RF_DR_40_SUDHA.pdf
Media
- extracted text
-
B
e
RF_DR_40_SUDHA
Delta
J
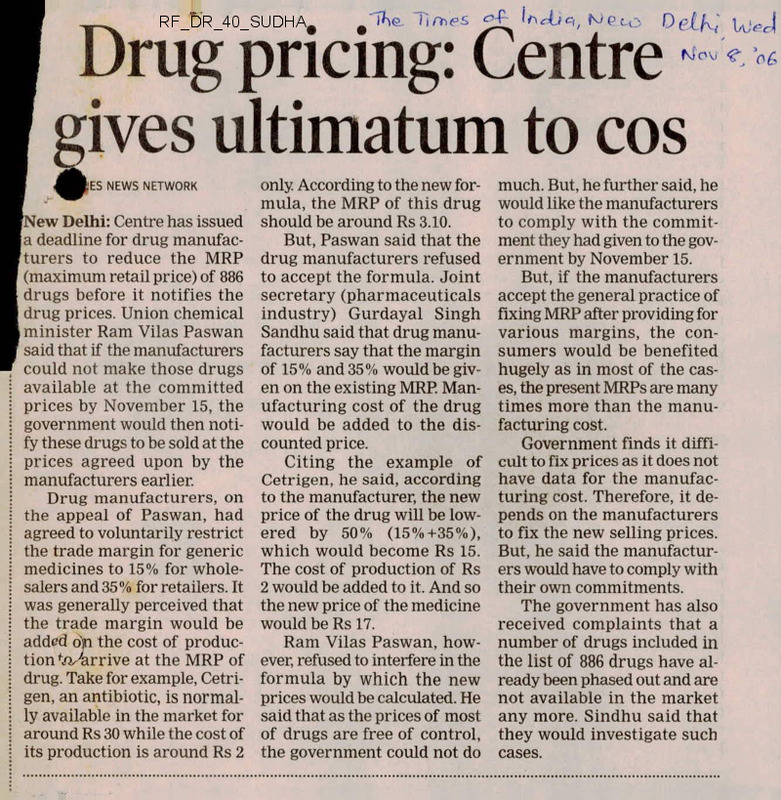
I Drug pricing: Centre •*-*-«
r gives ultimatum to cos
I
NEWS NETWORK
[New Delhi: Centre has issued
I a deadline for drug manufacturers to reduce the MRP
(maximum retail price) of 886
drugs before it notifies the
drug prices. Union chemical
minister Ram Vilas Paswan
said that if the manufacturers
could not make those drugs
available at the committed
prices by November 15, the
government would then notify these drugs to be sold at the
prices agreed upon by the
manufacturers earlier.
Drug manufacturers, on
the appeal of Paswan, had
agreed to voluntarily restrict
the trade margin for generic
medicines to 15% for wholesalers and 35% for retailers. It
was generally perceived that
the trade margin would be
added djn the cost of production <Oz4irrive at the MRP of
drug. Take for example, Cetrigen, an antibiotic, is normally available in the market for
around Rs 30 while the cost of
its production is around Rs 2
only. According to the new formula, the MRP of this drug
should be around Rs 3.10.
But, Paswan said that the
drug manufacturers refused
to accept the formula. Joint
secretary (pharmaceuticals
industry) Gurdayal Singh
Sandhu said that drug manufacturers say that the margin
of 15% and 35% would be given on the existing MRP. Manufacturing cost of the drug
would be added to the discounted price.
Citing the example of
Cetrigen, he said, according
to the manufacturer, the new
price of the drug will be lowered by 50% (15%+35%),
which would become Rs 15.
The cost of production of Rs
2 would be added to it. And so
the new price of the medicine
would be Rs 17.
Ram Vilas Paswan, however, refused to interfere in the
formula by which the new
prices would be calculated. He
said that as the prices of most
of drugs are free of control,
the government could not do
much. But, he further said, he
would like the manufacturers
to comply with the commitment they had given to the government by November 15.
But, if the manufacturers
accept the general practice of
fixing MRP after providing for
various margins, the consumers would be benefited
hugely as in most of the cases, the present MRPs are many
times more than the manufacturing cost.
Government finds it difficult to fix prices as it does not
have data for the manufacturing cost. Therefore, it depends on the manufacturers
to fix the new selling prices,
But, he said the manufacturers would have to comply with
their own commitments.
The government has also
received complaints that a
number of drugs included in
the list of 886 drugs have ai
ready been phased out and are
not available in the market
any more. Sindhu said that
they would investigate such
cases.
I have added points 9 and 10.
If somebody can simplify this (Naveen?), it can go as a press release.
Chinn
Dear friends,
We share with you certain disturbing developments with regards to Drug
Policy which need to be protested against strongly.
Drug Policy and Pricing: Putting the real issues in cold storage. ,Why
these Token Measures Won’t Work
The Government and Mr. Paswan are,announcing measures on 2nd October,
to reduce the prices of medicines. These, are mere sops designed to divert
public attention from the deals being made behind the scenes, and the
calculated delays in announcing the pricing component of the new Drug
policy.
1. The price control component of the policy has been deliberated upon by
committee after committee over the past few years. The Drug Price Control
Review Committee in 1999 toured Europe to discover that price control
exists in virtually all other countries. The pharmaceutical policy in 2002
planned to do away with price control altogether restricting it to less than 30
drugs. A Karnataka High Court Judgement stayed the implementation of the
policy and the final hearing of the case in the Supreme court is still pending.
The various committees appointed by the Government have been in response
to the litigation rather than in response to public interest. The Sandhu
Committee in 2004 was appointed to look at price control mechanisms and
recommended price control over entire categories of essential drugs, which
would have been a welcome step. A task force was later appointed in 2005,
chaired by Dr. Pronab Sen to look at mechanisms other than price control to
ensure availability of drugs at reasonable prices. It mentioned
(uncomfortably for the industry) that price regulation was the only credible
deterrent for the industry. In 2006, we were told about a new policy
formulated by the Ministry after a wide process of consultation and taking
into account the suggestions of the various committees. This policy talked of
regulating the prices of the 354 essential medicines in the National List of
Essential Medicines , which had been submitted to the Cabinet for approval.
This was followed by loud protests not only by the drug industry but also by
the Finance Ministry, and the Commerce ministry. The Health Minister has
maintained a eloquent, sphinx like silence. That India has the highest
number of people in the world who lack access to essential medicines,
because the government does not provide them and the people cannot pay for
them, doesn’t apparently bother^him. After all he has nothing to do with
Deleted: Sops and Tokenism by
Governments]
[ Deleted: is
[Deleted: ,
i Deleted: which
formulation of the Drug Policy, which is formulated by the Ministry of
Chemicals!
I
2. In a democratic country, such loud and vehement protests by the industry,
adequately represented in the media, have obviously to be paid heed to. So
the Ministry of Chemicals has made a 14-member committee to look into the
matter of price regulation again. Of these ll members are from the Industry^
and 3 from the government. This brazen act is a valuable precedent for the
future of public policy making in India; forest policy by timber merchants
association, cement and steel policy by cement manufacturers, housing policy
by Ansals and DLF, retail policy by Ambani and Wal mart, revision of the
Indian penal code by those indicted by law, etc.
3. The central issue with regard to Drugs in India today is their pricing. The
pricing of drugs determines directly which kind of drugs are manufactured,
promoted, and prescribed in the country. And we know that that overpriced,
non-essential, irrational and drugs sell more in this country.1
4. Regulation of the prices of a small number of drugs is the only visible kind of
regulation of the drug industry. The majority of the drugs are outside this list
of price control. In this very price-decontrolled segment, there are huge
variations, explained only by rampant profiteering. J'he government has been
allowing companies to market drugs for mental illness which cost more than 15
times, antibiotics and anti-cancer drugs which cost more than 10 times, and
drugs for diabetes and hypertension which cost more than 5 times other
competitive brands leading to huge windfalls for the companies. Does the
industry or the Ministry of Chemicals, the Finance, Commerce Ministry and the
Prime Minister’s office which are so vehement in their opposition to price
regulation have any credible explanation for this phenomenon? The
Government refuses to recognise, because it doesn’t want to, a fundamental
fact known the world over. A free market does not exist in drugs, where the
fundamental choice of the product is not decided by the consumer but by
doctors, who are heavily influenced by the companies to choose more
expensive preparations. The state of distress under which patients are forced
to purchase medicines does not allow them the freedom to not buy the
products either. A free market means in reality the freedom to overcharge
consumers by exploiting their ignorance and vulnerability and denying the
government any right to intervene.
5. On paper the government is supposed to monitor the prices of the drugs
outside price control and clamp price control wherever the behavior is
abnormal. Yet when the attention of the Chairman of the National
Pharmaceutical Pricing Authority was drawn to the brazen instances of
overpricing, he responded by making the extraordinary point that even in
[ Formatted
[Deleted; se
I
the case of a fdrug which is priced 14 times its competitor, he cannot
intervene unless it can be shown w show that the annual rise in its price was
greater than 20%.
[ Deleted: we can
6. The effort of the pharmaceutical industry is that retail drug prices, should
not be questioned at all, and they should be left to the ‘free’ market.
According to it, drug prices are so finely balanced at the moment that any
regulation would spell catastrophe for the industry. In the current policy,
regulation of a drug’s price means allowing a post-manufacturing allowable
margin of 150-200%
7. The variations in the drug retail prices have already been mentioned. The
industry cannot explain how it is possible for 2 companies to make the same
drug and sell at 10 times the price difference. The industry cannot explain
how it can sell a drug or an injection at a catastrophically low price of even
10% of the retail price , and afford it and yet complain if any attempt to
lower the final retail price to the consumer? 2The industry should explain
howjt sells J drugs to the government at even 5% of the retail price, and
afford it , and , and yet paint these pictures of doom if the MRP is
rationalised? The industry should explain how it can afford to ply doctors
with gifts, cars and air conditioners, host five-star conferences and spend
more than Rs. 5800 crores on drug promotion alone. The reality is that there
is a huge differential between the cost of manufacture of a drug and its retail
price, which is used for huge profits for the industry and trade and for
underwriting the costs of unregulated and unethical drug promotion. The
present largely unregulated system is to the benefit of everyone, except the
consumer who is either ignorant, or powerless.
The minister is putting the much awaited decision to introduce price control
into cold storage, and introduce an entirely token set of steps. He has
constituted an entirely jllegitimate committee made up almost entirely of
industry representatives to deliberate on price control?_,The Tamil Nadu and
Delhi state models of selective tender and pooled procurement have clearly
demonstrated that the drug availability in the public health system can be
vastly improved without massive increases in budget. But rather than talk
about such measures, we are being offered district level drug banks based
on so-called charity by drug companies. In return perhaps for getting the
spectre of price control off their backs, and putting the matter into deep cold
storage, they shall donate medicines worth 0.5% of their turnover, even as
drugs shall continue to be priced at 1000% of their cost of manufacture.,
8. The proposed measure of putting a cap on the margins of, drugs (that is*
those sold under generic names only) which do not comprise any significant
part of the market, is another attempt at denying the truth about drug
prices and obfuscation. The new policy polices the prices of generic-generic
drugs and branded-generics, while leaving the prices of branded drugs
intact. We are being told of upto 70% reduction in the prices of some drugs.
( Deleted: sei
[ Deleted: afford to
Deleted: illegitimate
committee
Deleted:,
Deleted: which they shall do
perhaps over the next ter years.
Deleted: Rather than
implement measures to improve
drug availability in the public
health system
Deleted: based on so-called
charity by drug companies . For
the people in distress, all that is
required now is that they should
be living on the right side of the
poverty line, and should be
certified as living so, and when
they fall ill, send their relative
straight to the district level drug
bank, where drugs contributed
by the industry out of 0.5% of
their turnover shall be available
for their consumption. There
may be of course some delay in
the entire process, but perhaps
his cabinet colleague shall oblige
by announcing free train travel
for those travelling more than
100 km to procure drugs for
their ill relatives. Shall these
banks be open after office hours,
or shall there be automatic drug
dispensing machines? 51
Formatted: Bullets and
Numbering
Deleted: only generic
Which are these drugs, and what is so special about them? And what about
the others, whose prices are not going to come down? These drugs we are
told are the ones which are generic-generic and branded generic.
___ Generic drugs are drugs which are sold under their non-proprietary
name, and not under the trade name. These drugs in India are only a
miniscule part pf the market. JVe challenge the Minister to point out generic
preparations are available easily for the treatment of anemia, tuberculosis,
dehydration, ^hypertension, diabetes or cancer, and are prescribed and being
sold. There are none.
We would ask the Hon 'ble Minister to define in legal terms what is being meant
by branded generics ( a complete misnomer if ever there was one). Can he point
out any such preparation which does not have a brand name ? Then how can it
be a generic?
The so-called branded generics are merely brands which are being promoted
to the chemists. They offer the clearest evidence of the kind of overpricing in
brands that is being allowed, and the fact that the prices of ALL drugs can
be brought down substantially without affecting the reasonable profitability
For example in the case of ciprofloxacin, the retail price of the leading brand
——fc-around Rs. 9 for a tablet of 500 mg. The Delhi state procurement price
for the same drug made by another company , found to be of good quality, is
10 times less. Now another reputed company promotes the same drug under
a_brand B offering it to the trade at Rs, 1.40 paise, but with a MRP of Rs. 7,
Anyone can understand that the conclusion to be drawn is that the MRP of
Rs. 7 or Rs. 9 should be questioned because it is clearly an inflated one and
that should be regulated. However our Government draws a different
conclusion and finds fault with the 400% margin, and is chastising brand B,
while leaving brand A intact. Why should there be a different benchmark for
the price of two brands? Is the government admitting to differences in
quality?
These so-called branded generics have grown as a segment, but still
constitute a tiny minority of the pharmaceutical trade,
JVe challenge the Government to point out a single instance of a branded
generic being a part of the top 300 brands, the sale of which itself accounts for
Rs. 18,000 crores.
---- The drug policy is silent on many issues which are of crucial concern to
the citizens of this country. The policy is silent on the issues emerging from
TRIPS and what safeguards will be used and when to ensure affordability of
drugs. Poor Indians are being converted into guinea pigs for the world in
clinical trials which are being conducted flouting all norms of consent, ethics
and safety. The policy is silent on the issue of irrational drugs and hazardous
—LU_ the Indian market, which no self-respecting drug regulatory
authority in the world would approve of. The draft policy part A, mentions
[ Formatted
[ Formatted
Formatted
)
plans to make India the drug maker of the world, but does not wish to follow
world standards of what constitutes a rational or safe drug. Drug promotion
in_India is completely unregulated and a major contributor to the inflation of
drug prices, , is increasingly turning into bribing to prescribe. There is a
complete lack of availability of unbiased prescribing information, lack of
norms or regulation with regard to prescription quality, and lack of
regulation over the kind of dispensing provided by India’s chemists.
jBut first, the the government’s bluff needs to be called on the issue of drug
prices. ¥
We call upon all members of civil society and concerned citizens of India to
use the means at their disposal to draw attention to the continued cheating of
the consumer in the area of drug prices,, represent matters in the media, ask
Members of Parliament to raise questions on drug policy and the nature of
this committee.
If a common program of protest can be organised in New Delhi over these
developments, AIDAN shall be willing to put its weight behind it
In Solidarity,
All India Drug Action Network.
The top selling brand in India is Corex, a widely abused cough expectorant containing opiods.
Nimesulide, a hazardous drugs, sells more than Ibuprofen. Dexorange with a composition that any
preparation for anemia should never have, is the top selling preparation for anemia. In a country where
chronic hunger is the lot of the majority of the population, appetite stimulants and nutritional supplements,
which cost more than almonds sell for hundreds of crores
‘ 2 years ago, Mr. Paswan announced that the trade margins in the case of 3 drugs investigated by the
Ministry, Nimesulide, Omeprazole, and Cetrizine, were over 1000%. 2 years later, and much media
publicity later, they remain to be so.
[Deleted:
[Deleted: d.
— Original Message —
From: Mira Shiva
To: drdabade@qmail.com
Cc: amol p@vsnl.com ; Prasanna Saliqram
Sent: Sunday, October 01,2006 6:54 PM
Subject: Fwd: Re: drug policy
Mira Shiva <mirashiva(d),yahoo.com> wrote:
Date: Sun, 1 Oct 2006 05:31:25 -0700 (PDT)
From: Mira Shiva <mirashiva@yahoo.com>
Subject: Re:
To: Anurag Bhargava <madhurag bhargava@jediffinail.com>. Anant Phadke <cerd@satyam.net.in>
CC: Dabade Gopal <dabade pal@,yahoo.com>. Chinu <sahajbrc@icenet.net>
Dear Anurag,
The OPPI, IDMA ,FICCI ,CII,Commerce Ministry , Consumer Affairs Ministry ,
Finance Ministry , Planning Commission PM's Office Including the Health Ministry
are all against Price Control. Consumer Affairs & Commerce ministry have objected to
price control because it would negatively affect FDI.
AIDAN.s note against Paswan may strategically not be appropriate may weaken the little
stand on price control that is being taken . We could make supportive statement on Price
control. The Finance Minister has said that decreasing Excise duty would mean
REVENUE LOSS " Paswan is trying to get 50% decrease in excize We should support
decrease in Excize duty on Duty on drugs that are essential & RATIONAL drugs
.NOT FOR NONESSENTIAL .IRRATIONAL DRUGS etc
The 14 member group by Chemical's Ministry to negotiate with the Industry was made
at the behest of PM's Office .Some of the companies have made some positive OFFERs
.the catch would be in what they wangle out of the Govt as a bargain ..
The Data Exclusivity for 5 years was pushed mainly by Dr Mashelkar.
In the AIDAN statement we should add something about the drug policy
ESSENTIALITY & RATIONALITY of the drugs in the market
ACCESS , (EQUITY .DISTRIBUTIVE JUSICE )
QUALITY SAFETY
AFFORDIBILITY
UNBIASED DRUG INFORMATION TO DOCTORS & CONSUMERS
ETHICAL MARKETING PRACTICES
RATIONAL USE OF DRUGS
We should not presume people remember this & it is also important to remind everyone
that THE PHARMACEUTICAL POLICY SHOULD COVER MANY ASPECTS
BESIDES DRUG PRICING .For a country with poor public health services ,80 % drug
purchase of medicines OUT OF POCKET thr NATURE OF DRUGS in the market
becomes all the more important, when the nature of diseases afflicting our people are
Acute Communicable diseases as well as chronic diseases requiring long term tratment
For various reasons the voice ofthe consumer , & peoples voice has been hardly audile .
There are some genuine constraints & some we have created for oursemves .
Anurag do not even wait for JSA , this could with the few additions could go as
AIDAN .
Anurag Bhargava <ma(lhurag_bliargava@rediffmaU.com> wrote:
dear friends,
here is a possible piece from AIDAN which with some modification of content could go on the JSA eforum. Perhaps a press release too can be made,
do send comments.
anurag
Anurag and Madhavi Bhargava
HIG-B 12 Parijat Extension , Nehru Nagar , Bilaspur- 495001 , Chhattisgarh Residence07752 270751/519276
JSS Centre: between 9.30 a.m.to 6 p.m.: 07753 244819
9. The proposed measure of putting a cap on the margins of only generic drugs
(that is those sold under generic names only) does not meet the problem head
on. These generic drugs are only Rs 2000 crores of a total market of Rs
30,000 crores and more. And most (approx 62 percent) of the top-selling 300
drugs in the ORG-Nilelsen Retail audit list are irrational and overpriced. So
can we first get rid of these irrational medicines?
10. The pharma industry does not directly lose if prices of drugs are capped only the trade loses its hefty margins. So the argument that the pharma
industry will suffer has no basis.
Mr. Paswan’s and the government’s bluff needs to be called.
We call upon all members of civil society and concernd citizens of India to use
the means at their disposal to draw attention to the continued cheating of the
consumer in the area of drug prices, represent matters in the media, ask
Members of Parliament to raise questions on drug policy and the nature of this
committee.
If a common program of protest can Ibe organised in New Delhi over these
developments, AIDAN shall be willing to put its weight behind it.
In Solidarity,
All India Drug Action Network.
How are drug prices controlled?
Monday, July 10, 2006, The Financial Express
In keeping with the Supreme Court ruling that all life-saving drugs should remain under price control, the
Union chemicals and fertilisers ministry has drafted a new pharmaceutical policy, proposing to bring most
of the 354 “essential drugs” under price control, in addition to retaining the 74 drugs currently under
control. The SC order had come in response to an earlier policy formulation which proposed to remove the
“rigours of price control” through a reduction in the span of price control. Therefore, the latest move by the
ministry which is yet to be endorsed by other government departments and the Union Cabinet, marks a
reversal of the policy direction. Significantly, the ministry’s move also comes with some con-cessions, fe
takes a Closer Look at the evolution of the country’s drug pricing policy:
How many drugs are under price control and how are their prices controlled?
There are 74 bulk drugs (active pharmaceutical ingredients) and all formulations (medicines as we consume
them) containing one or more of these bulk drugs come under price control. The National Pharmaceutical
Pricing Authority (NPPA) determines the ceiling prices for controlled bulk drugs in intra-industry
transactions and the retail ceiling prices of controlled formulations. This is done under the Drug Price
Control Order (DPCO), 1995, issued under the Essential Commodities Act (ECA).
Ceiling prices are fixed as per a formula that gives 100% mark-up on ex-factory cost of the formulation.
The mark-up covers the manufacturers’ margin and trade margins. There are over 3,500 formulations
containing controlled bulk drugs and so are under price control. These include branded and unbranded
formulations. This is just a fraction of the around 800 bulk drugs and about 60,000 formulations consumed
in India.
In terms of span of price control, the latest estimates say less than 25% of the retail pharma market of Rs
25,000 crore is under control. The constitution of the controlled market in the overall market is considerabh'
larger than their numerical share because price-controlled drugs are mass consumption drugs.
On what basis were the 74 bulk drugs picked for price control?
The drug policy 1986 as modified in 1994, following which DPCO 1995 was issued, prescribed mass
consumption and absence of competition within the therapeutic segment as the price control criteria. In
2002, the government announced a new pharma policy. But the policy’s pricing component was held
invalid by the Karnataka high court which said it violated the spirit of the ECA by proposing to liberalise
price control.
What is the price control criteria in the pharma policy proposed in 2002?
Under it, a bulk drug would invite price control if its moving annual total (MAT) value is over Rs 25 crore,
or if any single formulator has a market share of 50% or more, or if MAT value is between Rs 10 crore and
Rs 25 crore and any one formulator has a market share of 90% or more. Mass consumption and single
player monopoly are tlie criteria, as in the previous (operational) policy, only the thresholds change. If these
criteria were implemented as per the April 2002 market figures, just about 35 drugs would have remained
under price control.
The Supreme Court, however, upheld the high court views and asked the government to formulate a policy
to keep all essential drugs under price control. The government then appointed two committees to examine
the issue of drug pricing: the GS Sandhu committee and a task force headed by Pronab Sen. It also prepared
a national list of 354 essential drugs.
What did these panels say?
The Sandhu panel proposed caps on wholesale and retail trade margins—10% and 20%, respectively, for
branded drugs and 15% and 35% for unbranded ones.
The Sen panel, mandated to suggest ways other than price control to reduce drug prices, said the prices of
314 essential drugs should be regulated through ceiling price based on the weighted average of the top three
brands by value. It argued against price control on bulk drugs and unbranded formulations and said
essentiality should be the sole criterion for price control. It also recommended compulsory pre-marketing
price negotiation for patented drugs.
What does the latest policy draft say?
It proposes to bring almost all the 354 essential drugs under price control, besides retaining control on the
74 DPCO drugs. The draft policy proposes continuance of cost-based price control. However, there won’t
be fixation of prices of bulk drugs as at present.
The rigorous inspection-based system for costing of bulk drugs would be replaced by a liberal system based
on market data. Also, Mape would be increased to 150% from 100% in case of controlled drugs. As for
drugs developed out of indigenous R&D, Mape would be even higher at 200%.
http://www.financialexpress.com/fe_full_story.php?contentjd=l33363
People's Democracy
(Weekly Organ of the Communist Party of India (Marxist) Vol. XXV No. 13 April 01, 2001
Decontrol of Drug Prices
Amit Sen Gupta
THE wolves are baying at the door, once again calling for further decontrol in the prices of drugs. Since
comprehensive price controls were imposed on drugs in 1979, drug companies have continuously
clamoured for their removal. It is a measure of the clout that these companies exercise, that over the years
they have regularly managed to extract their pound of flesh from the government. Thus successive price
control orders of the government have whittled down both the span of price control and the limits on
profitability.
Thus, in 1979, a total of 343 drugs—accounting for 85 per cent of drugs in the market—were placed under
price control. Profitability allowed on price controlled drugs ranged from 40 per cent to 75 per cent. In 1987
the number of controlled drugs were reduced to 166 - covering 60 per cent of drugs in the market, and
profitability allowed was increased to a range of 75-100 per cent.
In 1995 the number was further reduced to 74 - covering 35 per cent of the market, and profitability
allowed was hiked up to 15 per cent. At each point when price control has been reduced, there has been an
immediate spiralling effect on the prices of drugs. And each time this has been preceded by loud wails from
the Pharma industry about declining profitability—a claim however that has never been borne out by the
health of the balance sheets of the pharma sector.
This time around, the tone for further price decontrol was set a couple of years back by the then Finance
Secretary Sri Vijay Kelkar, who publicly held forth on the need to free the industry of all price controls.
The recent announcement by Sri Yashwant Sinha in his Budget speech of further price decontrol of drugs,
hence, was on expected lines. It is expected that only a handful of drugs will now remain under price
control, and this is bound to fuel rise in drug prices. This time, though, the plea of poor profitability for drug
companies has not been used. Possibly because, given that all major news channels these days discuss share
prices at great lengths, it would have been difficult to conceal the fact that Pharma shares have remained the
most profitable even when there have been dizzy gyrations in the stock market.
Instead the rationale now being used to justify price control is two pronged—one that market forces are bsst
suited to stabilise drug prices, and two that the industry must be made more profitable in order for it to
increase investment on R&D and be globally competitive. Both these arguments are seriously flawed, and
are being used to justify the unjustifiable. Let us examine both these arguments.
DRUG PRICE CONTROL IS A GLOBAL PHENOMENON
It is important to underline that drug prices are controlled by differing mechanisms all over the world,
including in developed capitalist countries. In Australia since 1993, new drugs with no advantage over
existing products are offered at the same price. Where clinical trials show superiority, incremental cost
effectiveness is assessed to determine whether a product represents value for money at the price sought. In
Britain, there exists the pharmaceutical price regulation scheme - a voluntary agreement between Britain’s
Department of Health and the Association of the British Pharmaceutical Industry in which companies
negotiate profit rates from sales of drugs to the National Health Scheme.
Globally, Drug Companies are being forced to reduce the cost of medicines. Pressure is being mounted by
Health Insurance Cos, Health Management Organisations (HMOs) and governments (in countries like UK
and Canada where the State provides Health Insurance cover) all over Europe and North America. These
pressures have become stronger in recent years with the realisation that spiralling Drug costs are making
Health insurance cover (whether state funded or privately managed) unsustainable. In all these countries
there is a major move to insist on generic prescription in most cases, thus opening up a huge generics
market. Large TNCs are forced to compete on more or less equal terms which a large number of lesser
known Cos, and also sell drugs at relatively cheaper rates. In the US, for example, from 1995 through 1997,
generic (i.e. drugs without brand names that are produced by small companies and are cheaper) drug prices
showed a double-digit rate of decrease. This shift was facilitated by the Hatch-Waxman Act, which made
the approval process of generic drugs much easier. Since 1984 this has resulted in a dramatic increase in
competition from generic drugs, leading to an estimated saving of 8-10 billion dollars in 1994 alone.
The fact that drug prices are controlled all over the world flows from the global experience that market
mechanisms cannot be expected to stabilise prices. Various other interventions are needed to manipulate the
market, in order to guard against monopolies emerging. Unlike in the case of consumer goods, there is no
direct relation between the market and consumers in the case of drugs. Drugs are purchased by consumers
on the advice of doctors or chemists.
Consequently, the marketing strategies of drug companies target doctors or chemists. Doctors are not
known to take decisions based on price of contending brands. Similarly chemists have no interest in selling
cheaper brands. So, if we believe that drug prices will be kept low by market competition, it is a belief that
is not borne out by the past experience, in India or elsewhere.
Here, it is necessary to nail another lie. There is a prevailing myth that drug prices in India are the lowest in
the world. This is at best a partial truth. Drugs, which are still Patent Protected, are much cheaper in India
due to India’s earlier Patent Act. It should be obvious that we will lose this advantage after amendment of
the Indian Patent Act of 1970. But off-Patent Drugs (which anyway account for 80-85 per cent of current
sales in the country) are not necessarily cheaper in India. In fact, generally, Drug prices for these Drugs are
higher in India than those in Sri Lanka and Bangladesh. In fact prices of some top selling drugs are higher
in India than those in Canada and the UK. Thus, clearly, the benefits of the advantage that the Indian Drug
Industry enjoys over all other Third World nations, in terms of the availability of indigenous technology and
a large domestic market, have not been passed on to the consumers.
FALSE PROMISE OF GREATER R&D ACTIVITY
Let us now turn to the argument that price decontrol is necessary to spur R&D activities in the drug
industry. When legitimate concerns were raised that amendment of the Indian Patents Act would result in
rise in Drug Prices, the Ministry of Chemicals and Fertilisers had consistently claimed that any rise in prices
would be kept in check through mechanisms in the Drug Price Control Order. It is extremely surprising that
now that amendments are being made in the Indian Patents Act, we should be simultaneously talking of
diluting Price Controls-. Any further dilution would mean virtual abandonment of Price Controls. If the
government is to consider this, under the garb of encouraging R&D, it will only substantiate earlier fears
that a change in the Patents Act can only lead to a spiralling rise in prices of drugs.
Present investments on R&D in the Drug Industry is less than 2 per cent of sales. The dubious logic that
price controls have led to this situation has been put forward. In the past two decades the span of price
controls has come down from in excess of 85 per cent of the Industry’s turnover to around 35 per cent. If
reduction in price controls is to spur R&D activity, why has there been no rise in R&D expenditure in the
past decade. It may be recalled that the 1995 policy had a provision for keeping all drugs developed by
indigenous R&D outside price controls for ten years. This too does not seem to have spurred any significant
R&D activity in the Industry. The issue of Price Controls has nothing to do with infrastructure development
for R&D, and the two issues need to be dealt separately. It appears as though the issue of R&D has been
used as a red herring by drug companies to lobby for price decontrol and thereby licence to profiteer.
A major constraint for the Drug Industry in India is the relatively small domestic market (compared to our
population). The solution to this constraint cannot be sought within the industry, as it has to do with the
extremely low purchasing power of over 80 per cent of our population. The belief that it is possible to
extract significantly larger amounts of’’surplus" as profits from the domestic industry, that can be
channelled for R&D, is thus fallacious.
WHY DO WE NEED A DRUG INDUSTRY?
Finally, we need to understand that drugs are a commodity that are required most crucially by those who are
least likely to be able to pay for them. Unlike commodities like cars or washing machines, the whole logic
for the existence of the Industry lies in its ability to provide its products to the people who are economically
deprived. If the Industry fails in this fundamental endeavour, the very reason for its existence is open to
question. We already have a situation where a majority of our population does not have access to drugs,
because they cannot afford to pay for them. In such a situation rise in drug prices can only "cost out" larger
sections of the population. It can then legitimately be asked, if those who require drugs the most are going
to be unable to afford drugs, why have a drug industry at all? The industry argues that adequate
competition, even in the absence of price controls, can peg down drug prices. If that is so, why are they
afraid of price controls?
http://pd.cpim.org/2001/april01/aprill_snd.htm
Few takers for drug cos’ claim on prices
P.T. Jyothi Datta
The Hindu Business Line, Monday, Feb 11, 2002
ATTEMPTS by pharma companies to allay fears of drug price rise on the ground that the market would be
the leveller are not gaining currency with consumers.
A reduced price control regime being imminent, following the recent Drug Policy 2002, consumer forums
are not buying the arguments by pharma majors that competition would stabilise the cost of medicines.
Drawing parallels from the inflationary trends in the basket of essential drugs, following the announcement
of the Drug Price Control Order (DPCO-1995) — the Delhi Science Forum (DSF) is sure of a price
escalation in medicines, this time around too.
Dr Amit Sengupta of DSF told Business Line, "The revised DPCO will be followed by similar inflationary
trends, since the principles applied to reduce the span of control are the same.”
Citing from the insights of a DSF study post-DPCO (1995), the DSF discounts the theory of increased
research and development (R&D) investments by pharma companies following reduced controls.
"In the past, price controls were slashed from 166 drugs to 74. In the last decade, it was diluted to about 30
per cent of the market to spur R&D activity. But R&D investments in the drug industry is still less than 2
per cent of sales," he said.
On the market ironing out prices, the DSF observed that since drug companies targetted doctors and
chemists to sell, the latter were under no compulsion to sell the lowest selling drug to the consumer. "The
market is too rigged and flawed to be seen as leveller," an analyst observed.
The DSF study also revealed that the top selling brand in a particular formulation was not the cheapest one
as borne out by a comparative cost of top selling drugs, as per the ORG Audit-Nov 1997.
It was found that Cifran (ciprofloxacin from Ranbaxy) cost 100.12 percent more than the cheapest brand in
its category.
Similarly, Norflox (norfloxacin from Cipla) and R-Cin (Rifampicin from Lupin) were 128.93 per cent and
163.52 per cent higher than the cheapest drugs in their segments.
A comparative study of drug prices between February 1996 and October 1998 found that the price increase
for drugs under price control was negligible, while prices for drugs out of control were up by an average
14.94 per cent.
Sporidex was up from Rs 54.25 in 1996 to Rs 61.10 in 1998; Digene was up from Rs 16.55 to Rs 27.10;
Crocin went up from Rs 3.89 to Rs 5.88. Using this as a touchstone, the DSF infers that medicine prices are
expected to increasingly and silently creep up, as against a one-time escalation.
The forum dispels as "myth" the contention that India had low drug prices. "It is higher than Sri Lanka,
Bangladesh, Canada and the UK", he said. In the UK and Canada, the health management organisations
moderated drug prices and in the countries, such as the US, the health insurance agencies acted as
arbitrators.
In India, however, the only regulating mechanism was on its way to complete dismantlement, come 2005,
in line with the WTO commitments, an analyst pointed out.
http://www.blonnet.com/2002/02/ 11 /stor i es/2002021101230100.htm
Pharmabiz.com
New Pharmaceutical Policy A savage attack on healthcare and a licence to profiteer
Friday, March 15, 2002 11:16 1ST
Amit Sen Gupta
The new Pharmaceutical Policy 2002 cleared by the Cabinet is a savage attack on healthcare in the country.
Though it has been termed as a "Pharmaceutical Policy", the new changes are only aimed at allowing a rise
in drug prices. This has been done at the behest of pharmaceutical companies, who have been given further
license to profiteer at the expense of the sick and the ailing. All other elements in the Policy are mere
w indow dressing to justify the price hike.
It may be recalled that in 1995 the number of Drugs under price control had been slashed from 166 to 74.
This had led to an immediate spiral in drug prices. The; new policy has further reduced the number of drugs
under Price Control to just 38.
Myth of Low Prices
There is a prevailing myth that drug prices in India are the lowest in the world. This is at best a partial truth.
Drugs that are still Patent protected are much cheaper in India due to Indians earlier Patent Act. It should be
obvious that we would lose this advantage after amendment of the Indian Patent Act of 1970. But off-Patent
Drugs (which anyway account for 80-85% of current sales in the country) are not necessarily cheaper in
India. In fact, generally, drug prices for these drugs are higher in India than those in Sri Lanka and
Bangladesh. In fact as Table 1 shows, prices of some top selling drugs are higher in India than those in
Canada and the U.K.
The above raises the important fundamental issues that the benefits of the advantage that the Indian
Pharmaceutical Industry enjoys over all other Third World Nations, in terms of the availability of
indigenous technology and a large domestic market, have not been passed on to the consumers.
International Cost Comparison of Drugs
BNF J
MIMS
(UK) |
(India)
”“£89''""“
Amoxycillin
250 mg
2.59
Ampiciiiin
250 mg
~ 3.18
2.42
Erythromycin
250 mg
2.87
3.28-4.17
Cephalexin
250 mg
7.74
4.46 _
IPropanolol
40 mg
0.25
1.39
Atenolol
50 mg
2.65
1.29
Prednisolone
10 mg
1.5
1.09
1.32
Paracetamol
500 mg
T.25..... . 0.32
Haloperidol
0.25 mg
0.13
1.6
0.55
”’ 0.5
Phenobarbitone
30
mg
______
0 25 T 0.28
BC ~ British Columbia Children"s Hospital Formulary
No.35, MarchT998
MIMS - MIMS India, March 1998
(Single units - tab./Cap./vial - has been taken fcTal^^
India Rupees (Rs.). Rough conversion rate is : 1 U.S.dollar = Rs.42.50 1
Canadian Dollar = Rs.25.00, 1 British Pound = Rs.70.00)
Drug Dose
BC
(Canada)
1.75
1.75
1.25
3.00
.... 1.25....
°-49
Single units - tab/cap/vial - has been taken for all drugs. Prices are in Indian Rupees. Conversion rateis
$1=42.52, 1 Canadian dollar = Rs25, 1 Pound = Rs 70.
Source: British Columbia Children”s Hospital Formulary, British National Formulary, No.35 March 1998
MIMS India, March 1998
Decontrol leads to price rise
In the New Policy, in one sweep, the volume of pharmaceuticals under price control has been reduced from
an estimated 40% to below 25% of the total drug market. There has been no attempt to provide even the
semblance ofjustification for the decontrol of drug prices. Earlier studies have clearly shown that prices of
drugs start rising as soon as controls are removed. This was evident in 1995-96, after the last round of price
decontrol effected through the Drug Price Control Order (DPCO) 1995. Further, in almost all segments, the
brand leader for a particular drug (i.e. the Brand with the highest turnover) is usually one of the most
expensive (in some cases twice as expensive!). This flies in the face of the argument that market forces and
competition stabilises drug prices. If a more expensive brand sells more in the market than cheaper
alternatives, it should be evident that the price of a drug does not determine its volume of sales.
This is so because market mechanisms are notoriously ineffective in stabilising prices of drugs, as there is
no direct interaction between the consumer and the drug market. Companies are able to sell over-priced
drugs through aggressive promotional strategies aimed at doctors and by providing lucrative margins to
chemists. 1 he Governmenf's claim, hence, that market forces shall prevent price increase is fraudulent. It is
even more surprising that pharmaceutical companies have been provided this windfall when even a lay
observer is aware that pharma stocks have been some of the most robust in the stock market.
Market mechanisms do not stabilise prices
It is precisely because of this phenomenon that practically all countries in the world have mechanisms to
control drug prices. Controls on Drug Prices are exercised in many Market Economy countries. In spite of
strong Patent Protection, there are effective measures in place that allow regulation f Drug Prices. In
Australia since 1993, new drugs with no advantage over existing products are offered at the same price.
Where clinical trials show superiority, incremental cost effectiveness is assessed to determine whether a
product represents value for money at the price sought.
In Britain, there exists the pharmaceutical price regulation scheme - a voluntary agreement between
Britain"s Department of Health and the Association of the British Pharmaceutical Industry in which
companies negotiate profit rates from sales of drugs to the National Health Scheme.
Globally, drug companies are being forced to reduce the cost of medicines. Pressure is being mounted by
Health Insurance Cos., Health Management Organisations (HMOs) and Governments (in countries like
U.K. and Canada where the State provides Health Insurance cover) all over Europe and North America.
These pressures have become stronger in recent years with the realisation that spiraling Drug costs are
making Health insurance cover (whether state funded or privately managed) unsustainable. In all these
countries there is a major move to insist on generic prescription in most cases, thus opening up a huge
generics market. Large TNCs are forced to compete on more or less equal terms with a large number of
lesser known companies, and also sell drugs at relatively cheaper rates. In the U.S., for example, from 1995
through 1997, generic drug prices showed a double-digit rate of decrease. In the U.S. this shift was
facilitated by the Hatch-Waxman Act, which made the approval process of generic drugs much easier. Since
1984 this has resulted in a dramatic increase in competition from generic drugs, leading to an estimated
saving Of $8-$l0 billion in 1994 alone.
Thus, it needs to be understood that market mechanisms alone cannot be expected to stabilise prices.
Various other interventions are needed to manipulate the market, in order to guard against monopolies
emerging.
Red Herring of R&D
I he new pol icy has attempted to justify the price decontrol with the plea that this shall boost R&D
expenditure in the pharmaceutical sector. When concerns (legitimate in our view) were raised that
amendment of the Indian Patents Act would result in rise in Drug Prices, the Ministry of Chemicals and
Fertilisers had consistently claimed that any rise in prices would be kept in check through mechanisms in
the DPCO. It is extremely surprising that now that we are moving towards a Product Patent regime (the
amendment to the patents Act ispresently pending in Parliament), there should be talk of diluting Price
Controls. Price Controls have already been diluted in the past decade and only 40% of the turnover of the
Industry was under Price Control prior to the new policy. Any further dilution would mean virtual
abandonment of Price Controls. If the Govt, is to consider this, under the garb of encouraging R&D, it will
only substantiate earlier fears that a change in the Patents Act can only lead to a spiraling rise in prices of
drugs.
Present investments on R&D in the Drug Industry are less than 2% of sales. The dubious logic that price
controls have led to this situation has been put forward. In the past decade span of price controls has come
down from in excess of 60% of the Industry"s turnover to around 30%. If reduction in price controls is to
spur R&D activity, why has there been no rise in R&D expenditure in the past decade? It may be recalled
that the 1995 policy had a provision for keeping all drugs developed by indigenous R&D outside price
controls for ten years. This too does not seem to have spurred any significant R&D activity in the Industry.
The issue of Price Controls has nothing to do with infrastructure development for R&D, and the two issues
need to be dealt separately. It appears as though the issue of R&D is being used as a "red herring” by drug
companies to lobby for price decontrol and thereby license to profiteer.
Millions "Costed Out”
Pharmaceuticals have another unique characteristic - those who need drugs most are the least likely to be
able to pay for them. Thus even a small increase in prices results in the "costing out" from the market of a
large number of people. In a country where half a million people die of Tuberculosis - a disease that can be
treated by over a dozen drugs - because drugs are unaffordable, such a license to profiteer is inhuman.
The imminent rise in drug prices comes at a particularly unfortunate juncture. The public health delivery
system is in shambles and large parts of it are being dismantled or privatised. Drug supplies at public health
facilities are at an all time low. This has already forced poor consumers to pay for medicines even if they
are being treated in public facilities.
Any further price rise can only push such patients to the brink of penury. It is significant in this context that
the Pharmaceutical Policy is announced by the Ministry of Chemicals and Fertilizers. Does the Ministry of
Health believe that Drugs are mere industrial products?
Not a Pharmaceutical Policy
Finally, it is a moot point whether the recent policy that has been cleared by the Cabinet Committee on
Economic Affairs can be called a Pharmaceutical Policy. A Pharmaceutical* Policy has to start with the
premise that Drugs are not like any other industrial products or consumer goods. Unlike say, washing
machines or cars, availability of affordable drugs may make the difference between life and death for
millions of people. A pharmaceuticalpolicy, thus, has to address the issues of quality, indigenous
manufacture, availability of essential drugs, review of existing irrational and hazardous drugs, and
affordability of drugs that are available. The new policy does not address any of these. An estimated 50% of
drugs in the market are irrational, or hazardous, or sub-standard.
De-industrialisation has increased in the drug industry at a frightening pace and many companies are
dependant on imported bulk drugs. Imports of finished formulations have increased by 420 per cent in the
past year! Clearly the new "policy" is only a ploy to allow profiteering at the expense of people's health.
The timing of the new policy is also significant - it has been announced when the country's Parliament is
not in session. It appears to be a deliberate, all too familiar attempt, to bypass democratic processes in the
country. It is hoped that the unjustified attack on people’s right of access to affordable medicines will be
debated in full in the coming session of Parliament.
http://www.pharmabiz.com/article/detnews.asp?articleid=l 1395§ionid=46
Liberal Pharma Policy for Whose Benefit?
Wednesday, April 24, 2002 I 1:16 1ST
Amit Sen Gupta
I couldn t help being both amused and disappointed by Mr. Gopakumar Nair"s reactions to my small note
on the New Drug Policy (Pharmabiz, March 21). Amused because the reactions are so predictable.
Disappointed because we would expect the "Indian" Industry, at least, to be more responsive to people"s
needs and peoples concerns. Drugs are not like cars or washing machines. In the case of drugs those who
need them most are the least likely to be able to pay for them. It is precisely because of this that pricing is
such a major issue. If high process cost out a majority of people from the market, there is no point in having
an indigenous pharmaceutical industry in the country.
International Price Comparison
Let me turn to the points Mr. Nair raises in his rejoinder. The first point is easily taken care of. Let me
assure Mr. Nair that 1 am aware that different countries in the world use different currencies. In my note the
note on conversion had been inadvertently missed. For readers who may have been impressed by words like
"misinformation" and "chaotic figures" used by Mr. Nair, the Table should read as follows: (see table
above)
That, I hope takes care of any confusion that may have been created and readers can draw their own
conclusions!
High Trade Margins Responsible for High Prices?
Mere statements asserting that high taxes, duties and trade margins are responsible for prices in drugs bein"
higher in India than in neighbouring countries are unacceptable. High taxes and duties on medicines is a
matter of concern in most developing countries. I would welcome an initiative by the Indian Industry, in
partnership with public interest groups, to press the Government for a review of the duty and tax structure.
However I would insist that the Industry also appear to be seriously interested in lowering drug prices by
taking other necessary measures. Mr. Nair talks of high trade margins. There are a large number of
instances where the same company sells generic drugs to wholesalers at one-fifth or less of the price at
which their branded products are marketed. The readers are free to draw their own conclusions as to who is
crucially responsible for hiking prices of drugs. May I underline here that the entire marketing system of
drugs in the country is designed to benefit BOTH the manufacturer and the wholesaler/ retailer. It is a
system that has been arrived at for their mutual benefit, disregarding the interests of consumers. The
industry is a captive to the demands of those involved in trading of medicines because it survives on
aggressive promotion of its products, and needs them for its own survival.
Misinformation on Low Drug Prices
Any confusion that may exist regarding rise in prices of patented and off-patent drugs is in fact an Industry
creation. It is the Indian industry that has for long asserted that Indian drug prices are the lowest in the
world - an assertion, unfortunately, that has been unquestioningly accepted by most. This is a contention
that we have always disagreed with. It is the Industry, which has repeatedly used figures regarding global
process of on-patent drugs to try to prove that Indian drugs are much cheaper. As my earlier note had
pointed out, this kind of selective use of figures deliberately hides the fact that off-patent drugs in India are
often more expensive, not only in developing countries, but aso in some developed countries. This is of
serious consequence because off-patent drugs account for more than 85% of the volume of drugs sold in the
country.
It is precisely because of this that we feel that tying up the issue of price-controls with change in the Patent
Act is illegitimate. What Mr.Nair is essentially saying is : allow us to make more profits (some would read
it as profiteer) and we will develop new drugs. It is because we had anticipated such an argument from the
industry that we had said that a change of the Indian Patent Act will be used as an excuse to decontrol drug
prices further and thus the amendment of the Indian Patent Act would lead to a hike in prices of off-patent
drugs too. Nr Nair"s assertions have only confirmed our worst fears in this respect.
Research and Development
Promising trends" notwithstanding the total R&D expenditure as a percentage of sales turnover has shown
little increase. Tying up price decontrol with the requirement to increase R&D expenditure suffers from the
pitfail that higher profits allowed to manufacturers do not necessarily translate into higher R&D
expenditure. We hold that in order to spur R&D activity the Government has to play a proactive role and
pledge resources for such activity. Global experience shows that private R&D expenditure always follows
public expenditure. We find the new drug policy entirely deficient in this respect - a 150 crore fund is a joke
for any country seriously considering the prospect of emerging as an R&D superpower!
Local Manufacture
I totally puzzled by the argument that price decontrol will promote local manufacture. Price decontrol
affects formulations - how will this promote bulk drug manufacture? Mr. Nair is of course correct (and
honest) when he says that an "open policy" helps industry. What he has not said is that it helps the industry
make profits and satisfy its shareholders. But then it would not require a genius to deduce that! But drug
manufacture, I assume, is done to remedy diseases. If drugs are too expensive for those who require them,
why do we need an industry at all?
WTO and the Industry
I am glad that Mr.Nair agrees with me on something - that the present situation has resulted in high import
dependency. I am not interested in even discussing how self-reliant we are in production of formulations.
India is not a Banana Republic, and I start from the premise that self-reliance means self-reliance in bulk
drug production. The question is not which government is responsible for the WTO regime that is in force.
The question is whether the government of the day and its policies are doing enough to protect indigenous
industry. Mr.Nair takes resort to the tired TINA (there is no alternative) slogan.
I fear that large sections of the Indian Industry are not even interested in manufacturing any more. They are
comfortable in being traders because their profits come from selling formulations, not from manufacturing
bulk drugs. Yes, the WTO regime has made the situation difficult for bulk drug manufacturers. But do we
see the same level of interest in this issue within the Industry as we see on the issue of price controls? We
need to seriously engage in a debate regarding how to counter the problem ofcheap imported bulk drugs.
Can we not think of a cell within the industry that works on anti-dumping measures? Can we not think of
measures to make bulk drug manufacturing more competitive?
Non-Intersecting Concerns
The last argument that Mr Nair has used really lets the cat out of the bag. He cynically asserts that the
situation of wide price variations for the same drug is something, " we must be prepared to deal with and
live with in an open market society". Who has to be prepared to do so? The Industry has no problems with
it, because it stands to gain from such instances of blatant profiteering. But the people of this country
certainly are not prepared to live with it. They, furthermore, do not require to be preached to on the merits
of an "open market society" by an industry that wishes to put profits over their misery. Clearly we are
talking of two non-intersecting sets of interests. I am disappointed because it need not necessarily be so. It
is possible for the industry and consumers to work together, at times, to their mutual benefit. But for that the
industry needs to show some sensitivity towards the needs of consumers.
http://www.pharn-iabiz.com/article/detnews.asp?articleid=11400§ionid=46
‘We don’t need no price control’
Drug companies chorus against Cabinet’s prescription
Amit Shanbaug
Mumbai. The Indian pharmaceutical industry has expressed its extreme displeasure at a proposed policy
that aims to control drug prices.
Slated to come up before the Cabinet for discussion soon, the new policy has the CII National Committee
on Drugs & Pharmaceuticals concerned that if and when it is implemented, it would have a disastrous
impact on the pharmaceutical industry and even the country as money for R&D would be reduced.
Ajay Piramal, Chairman of the CII committee and also the Chairman of Nicholas Piramal Limited, stated
that drug manufacturers spend only 5 per cent of their turnover on research & development (R&D) while
their foreign and MNC counterparts spend 15 to 20 per cent on the same.
It is necessary to increase investment on R&D for making medicines at affordable prices to consumers,” he
said.
He also added that despite inflation, in the last four years, the cost of 539 drug formulations have actually
dropped by nearly 5 per cent due competition and increased research.
Indian prices lowest in the world
Ranjit Sahani, Managing Director of Novartis Limited, pointed out that the prices of drugs in India are
lowest and it should be left on competition to decide pricing.
Over the years, due to Excise Duty and Value Added Tax, our margins have been decreasing. Now if the
government puts in a price control mechanism, margins would be further decreased directly affecting and
‘demotivating’ R&D,” he said.
At present 74 drugs comprising of 30 per cent of formulations are under a controlled price policy. If the
new drug policy comes into force, 354 drugs comprising 80 per cent of formulations be covered.
A drug’s cost is only 15 pc of the price
According to Kewal Handa, Managing Director of Pfizer India Limited, the industry had given a lot of
options to the government in the past wherein they could have procured medicines at just 50 per cent of the
price for hospitals.
We are yet to get any confirmation on that,” he revealed while adding that drug prices make just 15 per
cent of the health costs in the country where as taxes, hospitalisation costs and transportation of the patient
adds up to remaining 85 per cent. “The remaining costs can be eliminated if a focused study is done on how
to procure effective drugs,” he said.
http://www.mumbaimirror.com/nmirror/mmpaper.asp?sectid=13&articleid=71 820062231362037182006223034640
Drug Companies Up In Arms Over New Pharma Policy
Wednesday, July 19, 2006; Posted: 12:09 AM
RTTNews) - The government of India firmed up its stand on drug pricing by re-emphasizing its proposal to
bring 354 formulations under price control from the current 74. The policy is likely to go for Cabinet's
approval soon, according to media reports.
The country s pharmaceutical industry, under the aegis of the Confederation Indian Industries, came
together to oppose the policy and asked the government not to proceed on the draft drug policy in the
present form.
Novartis, the Swiss group and Nicholas Piramal, an Indian rival, are leading the revolt against moves they
claim would strangle margins, discoursing expansion into rural areas and could affect quality.
Meanwhile, the department of chemicals, which takes care of pricing regulations for medicines and
pharmaceuticals, stressed that the prices of generic drugs would be monitored. It also said the government
would ensure that they maintain the prescribed trade margin of 15% for wholesalers and 35% for retailers.
Satwant Reddy, secretary, department of chemicals, announced that vaccines, biologicals and non-branded
drugs would be exempted from the price control regime. Similarly, drugs, which have a maximum retail
price of Re. 1 or less, and drugs and other medical utilities procured in bulk by hospitals, would also be kept
out of the price regime, she said.
http://www.tradingmarkets.com/tm.site/news/ASIAN%20MARKETS/309673/
19 Jul 2006
Expanding Price Control Detrimental for Pharma Companies: CII
Expressing concern over the proposal to expand price control from 74 to 354 drugs in the proposed national
pharmaceutical policy, industry chamber CII today said the step will prove detrimental to the growth of the
industry as well as the economy.
"Healthy competition between pharma companies already ensures reasonable pricing. Instead of affecting
the growth by over regulation, it would be better to allow market forces to determine prices," CII’s National
Committee on Drugs and Pharmaceuticals Chairman Ajay Piramal told reporters after a meeting with the
committee members here.
The committee suggested that the government should maintain 'status quo' on the current 74 bulk drugs
under cost based price control and effective monitoring of the National List of Essential Medicines (NLEM)
drugs to control any abnormal price increase.
It also proposed to allow government agencies to procure at a ceiling price of 50 per cent of MRP
voluntarily and provide adequate incentives for R&D oriented Indian companies.
"Any sudden regulatory shocks like increased price contro would be detrimental to the industry and the
economy," Piramal said.
Quoting independent studies, Piramal said that there had been an average five per cent decline in real terms
over the last four years and drug prices in India are among the lowest in the world.
He pointed out that product patent regime will impact the industry leading to higher investment requirement
for R&D.
"Large investments in R&D are critical for affordable medicines in the future," he said.
Source:PTI News http://www.medindia.net/news/view_news_main.asp?x= 12497
Drug Price Regulation: An Imperative and an Obligation in India - Dr, Anurag Bhargava,
S. Srinivasan.
Will the final form of the pharmaceutical policy again neglect the predicament of patients , the
Indian experience of the free market in drugs and the priorities of public health?
The current proposed policy and the arguments of the industry:
No policy relating to the health of the Indian people arouses as much interest in the media as the
pharmaceutical policy. The current policy, evolved possibly in response to a directive of the Supreme
Court and a stated commitment under the common minimum programme, seeks to increase the number of
drugs under price regulation. The pharma sector is astir and has been trying to score points in the media.
Few counterpoints are being offered to clarify the real issues at stake, hence this piece.
The industry creates innovative arguments each time there is any talk of price regulation. It used to talk of
closure of units earlier, now there are visions of increase in number of spurious drugs, decline in exports
decline in R&D, and barely concealed threats of scarcity of drugs if price regulation is put in place. These
arguments are specious. It has been a part of the pharma policy that any drug developed by indigenous
R&D, shall be exempt from price control for a period of upto 15 years. There have been no claimants to
the best of our knowledge, for this exemption so far.
The arguments for price regulation:
The purchase of drugs is a unique situation.
The pharma industry portrays medicines as being like other consumer goods and patients being like other
consumers. In the case of medicines, the choice is exercise by a doctor, and not by the consumer, who is
usually ignorant of their nature. The need for medicines is often immediate, obligatory, even life-long, and
has life and death implications. For such a critical and essential commodity, governments all over the
world, even in so-called market economies regulate the prices of all medicines while providing them,
paradoxically, as part of a highly socialised system of healthcare.
The high personal cost of disease and the consequences of deregulation of drug prices in the past.
On the contrary in India we have one of the most privatised system of healthcare. 83% of healthcare related
expenditure is borne by out-of-pocket expenditure made by people, most often by the poor who fall sick
more often. More than two thirds of this expense in outpatient illnesses is made on purchase of drugs. Only
13% of the chemical entities made in India are presently under price regulation. The no. of drugs under
price regulation fell progressively from 347 in 1977 to 146 in 1986, 74 in 1995 to a projected 25 or so
drugs in 2002. The 2002 policy which would have virtually done away with price regulation, was stalled
on the interventions of the Karnataka High Court, who ruled that this would make essential and life-saving
drugs out of the reach of ordinary people. This case is subjudice in the Supreme Court. In fact each episode
ot deregulation has been followed predictably with a dramatic increase in drug prices. In 1995 for example
the price of a preparation for anemia rose by 177% while the price of anti-TB drugs rose by nearly 90%.
According to the WHO’s World Medicines Situation report of 2004, an estimated 649 million people in
ndia more than any other country in the world, lack regular access to essential medicines. The
availability of drugs in the public health system is abysmal by the Government’s own admission. The
increase in healthcare costs, of which drug price deregulation is a major cause has resulted in an increasing
number of people not seeking healthcare at all. Those who do seek healthcare fall into the trap of medical
poverty. Healthcare costs are becoming a significant cause of rural indebtedness and liquidation of assets
across the country.
The anarchy of prices of drugs which are outside the price-controlled list.
A strong argument for governmental regulation is provided by the reality of prices of drugs, which are
outside the price-controlled list. 2 reputed companies manufacture the same chemical in the same strength
with a retail price difference of even more than 1000%.Aventis charges Rs.95 for a single tablet of an
antibiotic like Levofloxacin 500 mg, while Cipla charges only Rs. 6.8 for the same tablet. The same drug
or diabetes can be sold for Rs. 2 as well as Rs. 10. The companies are freely charging upto 5-6 times in
the case of a drug for hypertension, upto 15 times in the case of a drug for a psychiatric ailment, upto 18
times the price of a drug for cancer, without any logic, and what is disturbing, without any intervention
from the government. The true nature of the market and the insensitivity of doctors to the price of a drug
is revealed by the fact the costlier brands often sell the most.
The real cost of manufacture of drugs: Price regulation is fully compatible with profitability.
The real cost of manufacturing drugs is often a very small fraction of the retail price. This is revealed by the
prices of drugs in competitive tenders, by the trade margins that companies offer, and by the humungous
amounts they can afford to spend on drug promotion.
Internationally an Indian company created a stir when in 2001 it offered to sell quality certified anti-HIV
drugs at 3% of the price at which American companies sell them. At home in India, even in quality
conscious bulk procurement processes like in Delhi and Tamil Nadu, the tender rates of drugs are as low as
2-20% of the market rate, which would be unheard of in any other commodity. Cadila Pharmaceuticals bid
tor supply of a medicine for worms, Albendazole 400 mg tablets was a mere 22 paise, while its ZYBEND
brand sells for Rs. I 1.90 in the market. A drug for hypertension like Atenolol is procured at 12 paise by
Delhi State while in the market the same drug is sold for as much as Rs. 2.50.
Trade margins in pharmaceuticals can be astronomical. Dr. Reddy’s Nimesulide is priced at Rs. 2.90 per
tablet, while Cipla offers the same to its traders at 10 paise per tablet. An antibiotic injection like Amikacin
made by Alembic has a retail price of Rs. 64, while the retailer can buy it at Rs. 12.50 . Numerous other
freebies are given to the trade which include free drugs, consumer items, overseas trips and the like. The
pharmaceutical sector needs to explain to the public how it can afford to sell drugs at even 10% of their
MRP to wholesalers and not suffer from loss of profitability and yet complain bitterly whenever the MRP is
sought to be lowered by the government? The new policy talks of a 150-200% margin on the post
manufacturing expenses for drugs under price control. Surely such a profit margin is adequate for
profitability of any manufacturing enterprise.
The need for a comprehensive, balanced and rational drug policy:
It is possible to balance the public good with private profit in a pharmaceutical policy. Profitability of drug
companies can be ensured while protecting the people of India from overpricing. The government is
planning to put all the 354 medicines in the National List of Essential Medicines under price control. Past
experience suggests that in the light of price regulation, the companies switch to production and promotion
of drugs, often irrational or higher priced alternatives which are outside the list. The government should
pre-empt this by bringing all alternative drugs also at the very least under a scheme of price monitoring.
The policy should put in place firm guidelines on conduct of clinical trials in India, limit new drug
approvals to entities which clearly confer a therapeutic and cost advantage, remove irrational formulations
which comprise a major part of the market, ban hazardous drugs, ensure quality in manufacturing and
testing of drugs, evolve stricter codes on pharmaceutical promotion, mandate provision of unbiased
prescribing information, implement nationwide pooled procurement schemes on the lines of Tamil Nadu
and Delhi, and improve availability of drugs in the public health system.
It needs to be clarified that price regulation is a national policy matter and in no way incompatible with
TRIPS. In fact it is even a greater imperative under TRIPS.
The MNCs are once again eyeing the huge market, which India has to offer, and pressing for all kinds of
deregulation. The pharmaceutical sector in India, which owes its existence and its success to strong
Governmental support, is clamouring for the same. The Government and its committees are all aware of the
fact of the anarchy of retail prices, the rise in prices after deregulation, the high trade margins, and the
impact of healthcare costs on people. If it still does not act in the public interest it shall be deemed
complicit in the rising graph of people’s miseries.
It remains to be seen whether concerns for the health of the people, and their distress, or the health of the
stock markets will engage the government in its thoughts and be reflected in its actions.
(Dr Anurag Bhargava is a practising physician in a rural community health program, and S.Srinivasan is
involved in manufacture of drugs at low-cost for community health programs. Both are members of the All
25 years)1^
NetW°rk Which h3S be6n camPaigninS on drug ^ues and a rational drug policy for over
Drug Price Regulation: An Imperative and an Obligation in India - Dr. Anurag Bhargava,
S. Srinivasan.
Will the final form of the pharmaceutical policy again neglect the predicament of patients ,
the Indian experience of the free market in drugs and the priorities of public health?
The current proposed policy and the arguments of the industry:
No policy relating to the health of the Indian people arouses as much interest in the media as the
pharmaceutical policy. The current policy, evolved possibly in response to a directive of the
Supreme Court and a stated commitment under the common minimum programme, seeks to
increase the number of drugs under price regulation. The pharma sector is astir and has been
trying to score points in the media. Few counterpoints are being offered to clarify the real issues
at stake, hence this piece.
The industry creates innovative arguments each time there is any talk of price regulation. It used
to talk of closure of units earlier, now there are visions of increase in number of spurious drugs,
decline in exports, decline in R&D, and barely concealed threats of scarcity of drugs if price
regulation is put in place. These arguments are specious. It has been a part of the pharma policy
that any drug developed by indigenous R&D. shall be exempt from price control for a period of
upto 15 years. There have been no claimants, to the best of our knowledge, for this exemption so
far.
The arguments for price regulation:
fhe purchase of drugs is a unique situation.
The pharma industry portrays medicines as being like other consumer goods and patients being
like other consumers. In the case of medicines, the choice is exercise by a doctor, and not by the
consumer, who is usually ignorant of their nature. The need for medicines is often immediate,
obligatory, even life-long, and has life and death implications. For such a critical and essential
commodity, governments all over the world, even in so-called market economies regulate the
prices of all medicines while providing them, paradoxically, as part of a highly socialised system
of healthcare.
The high personal cost of disease and the consequences of deregulation of drug prices in the
past.
On the contrary in India we have one of the most privatised system of healthcare. 83% of
healthcare related expenditure is borne by out-of-pocket expenditure made by people, most often
by the poor who fall sick more often. More than two thirds of this expense in outpatient illnesses
is made on purchase of drugs. Only 13% of the chemical entities made in India are presently
under price regulation. The no. of drugs under price regulation fell progressively from 347 in
1977 to 146 in 1986. 74 in 1995 to a projected 25 or so drugs in 2002. The 2002 policy which
would have virtually done away with price regulation, was stalled on the interventions of the
Karnataka High Court, who ruled that this would make essential and life-saving drugs out of the
reach of ordinary people. I his case is subjudice in the Supreme Court. In fact each episode of
deregulation has been followed predictably with a dramatic increase in drug prices. In 1995 for
example the price of a preparation for anemia rose by 177% w hile the price of anti-TB drugs rose
by nearly 90%. According to the WHO'sWorld Medicines Situation report of 2004. an estimated
649 million people in India, more than any other country in the world, lack regular access to
essential medicines. The availability of drugs in the public health system is abysmal by the
Government's own admission. The increase in healthcare costs, of which drug price deregulation
is a major cause has resulted in an increasing number of people not seeking healthcare at all.
I hose who do seek healthcare fall into the trap of medical poverty. Healthcare costs are becoming
a significant cause of rural indebtedness and liquidation of assets across the country.
The anarchy of prices of drugs which are outside the price-controlled list.
A strong argument for governmental regulation is provided by the reality of prices of drugs,
which are outside the price-controlled list. 2 reputed companies manufacture the same chemical
in the same strength with a retail price difference of even more than 1000%.Aventis charges
Rs.95 for a single tablet of an antibiotic like Levofloxacin 500 mg. while Cipla charges only Rs.
6.8 for the same tablet. I he same drug for diabetes can be sold for Rs. 2 as well as Rs. 10. The
companies are freely charging upto 5-6 times in the case of a drug for hypertension, upto 15
times in the case of a drug for a psychiatric ailment, upto 18 times the price of a drug for cancer,
without any logic, and what is disturbing, without any intervention from the government. The
true nature of the market and the insensitivity of doctors to the price of a drug is revealed by the
fact the costlier brands often sell the most.
The real cost of manufacture of drugs: Price regulation is fully compatible with
profitability.
The real cost of manufacturing drugs is often a very small fraction of the retail price. This is
revealed by the prices of drugs in competitive tenders, by the trade margins that companies offer,
and by the humungous amounts they can afford to spend on drug promotion.
Internationally an Indian company created a stir when in 2001 it offered to sell quality certified
anti-IIIV drugs at 3% ol the price al which American companies sell (hem. At home in India,
even in quality conscious bulk procurement processes like in Delhi and Tamil Nadu, the tender
Kites <.)! dings are as low as 2-20% ol the market rale, which would be unheard of in any other
commodity. Cadila Pharmaceuticals bid for supply of a medicine for worms. Albendazole 400 mg
tablets was a mere 22 paise, while its ZYBEND brand sells for Rs. I 1.90 in the market. A drug
for hypertension like Atenolol is procured at 12 paise by Delhi Stale while in the market the
same drug is sold for as much as Rs. 2.50.
Trade margins in pharmaceuticals can be astronomical. Dr. Reddy's Nimesulide is priced at Rs.
2.90 per tablet, while Cipla offers the same to its traders al 10 paise per tablet. An antibiotic
injection like Amikacin made by Alembic has a retail price of Rs. 64, while the retailer can buy it
at Rs. 12.50 . Numerous other freebies are given to the trade which include free drugs, consumer
items, overseas trips and the like. The pharmaceutical sector needs to explain to the public how it
can afford to sell drugs at even 10% of their MRP to wholesalers and not suffer from loss of
profitability and yet complain bitterly whenever the MRP is sought to be lowered by the
government? I he new policy talks ol a 150-200% margin on the post-manufacturing expenses for
drugs under price control. Surely suchi a profit margin is adequate for profitability of any
manufacturing enterprise.
The need for a comprehensive, balanced and rational drug policy:
It is possible to balance (he public good with private profit in a pharmaceutical
pharmaceutical policy.
policy.
ITolnubility ol drug companies can be ensured while protecting the people of India from
overpricing. The government is planning to put all the 354 medicines in the National List of
Essential Medicines under price control. Past experience suggests that in the light of price
icgulation. the companies switch to production and promotion ol drugs, often irrational or higher
priced alternatives which are outside the list. The government should pre-empt this by bringing
all alternative drugs also at the very least under a scheme of price monitoring.
1 he policy should put in place firm guidelines on conduct of clinical trials in India, limit new
drug approvals to entities which clearly confer a therapeutic and cost advantage, remove irrational
formulations which comprise a major pan of the market, ban hazardous drugs, ensure quality in
♦
manufacturing and testing of drugs, evolve stricter codes on pharmaceutical promotion, mandate
provision of unbiased prescribing information, implement nationwide pooled procurement
schemes on the lines of Tamil Nadu and Delhi, and improve availability of drugs in the public
health s\siem.
h needs lo be elarilied lhat price regulation is a national policy matter and in no way incompatible
with TRIPS. In fact it is even a greater imperative under TRIPS.
The MNCs are once again eyeing the huge market, which India has to offer, and pressing tor all
kinds of deregulation. The pharmaceutical sector in India, which owes its existence and its
success to strong Governmental support, is clamouring for the same. The Government and its
committees are all aware of the fact of the anarchy of retail prices, the rise in prices after
deregulation, the high trade margins, and the impact of healthcare costs on people. II it still does
not act in the public interest it shall be deemed complicit in the rising graph of people’s miseries.
It remains to be seen whether concerns for the health of the people, and their distress, or the
health of the stock markets will engage the government in its thoughts and be reflected in its
actions.
( Dr. Anurag Bhargava is a practising physician in a rural community health program, and
S.Srinivasan is involved in manulacture of drugs at low-cost for community health programs.
Both arc members of the All India Drug Action Network which has been campaigning on drug
issues and a rational drug policy for over 25 years)
For Immediate Release
20 July 2006
MOVE TO BRING ESSENTIAL MEDICINES UNDER PRICE CONTROL HAILED
All India Drug Action Network (AIDAN) calls for a comprehensive, balanced and rational
drug policy
have
a
'
(A'DAN)’ 3 nati°nal netW0rk of a number °f organisations who
7 ACti°n
decision of rhrZnCh ^i0^11031
L Jo EsseX M X
MiniXy A OAN
J
X’
iSSU6S SinC6
” Fer“'i8erS “niS,ry “ bri"9
“n,r°L
eightieS' has hailed
,be ™aid"«
to
istry A DAN has said that in a country where over 60% of the population lack regular access
to essential medicines, the move was certainly in the right direction.
(H*
XpZXxxltj rhav,ns ’ —-
xr—
demand’ q
h community but on aggressive marketing techniques and created
from 347 ' Z?5176 9°Vernmenfs had brought down the number of drugs under price control
As opposed to the industry claims that 'price regulation would hit profitability AIDAN has said
AIDAN has stated that the trade margins in pharmaceuticals
done by VOICE, a consumer education organisation, andcan be astronomical’ A study
Pharmaceutical Pricing Authority, had shown that the difference i supported by the National
in price to the retailer and that to
the patient could well be over 400%. For instance, Nimesulide
tablets, while the price to the retailer was just Rs. 6 for the same. ! was available at Rs. 24 for 10
company spe„a a sma„ ,ractl0n on RSD as compa,ed t0 mark.ling and pJ01i(>n „
AM
However, AIDAN has warned that just bringing the essential drugs under price control was
not enough. With an estimated 649 million people in India lacking regular access to essential
medicines (World Medicines Situation Report, 2004; WHO), there is an urgent need to institute a
comprehensive, balanced and rational drug policy in the country. Some specific demands of
AIDAN are:
1) Increase the scope of the drug price control to include alternate drugs
Past experience suggests that in the light of price regulation, companies switch to production and
promotion of alternative, irrationally priced drugs outside the control list. The government should
pre-empt this by bringing all alternative drugs also under price control. If this is not found feasible,
they should at least be brought under a price monitoring scheme.
2) Guidelines on clinical trials
The policy should put in place firm guidelines on the conduct of clinical trials in India and limit
new drug approvals to entities that clearly confer a therapeutic and cost advantage.
3) Rational and safe drugs
The policy should mandate the removal of irrational formulations that comprise a major part of
the market, ban hazardous drugs, and ensure quality in the manufacturing and testing of drugs.
4) Drug promotion and availability
The policy should evolve stricter codes on pharmaceutical promotion; mandate provision of
unbiased prescribing information; implement nationwide, pooled procurement schemes on the
lines of Tamil Nadu and Delhi; and improve availability of drugs in the public health system.
AIDAN also made it clear that price regulation is a national policy matter and in no way
incompatible with trade-related intellectual property rights (TRIPS). In fact, it is an even greater
imperative to have price regulation under the TRIPS regime. AIDAN claimed that the
pharmaceutical sector needed to explain to the public how it could afford to sell drugs at even
10% of the MRP to wholesalers and not suffer from loss of profitability, and yet complain bitterly
whenever the MRP was sought to be lowered by the government. The new policy talked of a 150200% margin on the post-manufacturing expenses for drugs under price control. Surely such a
profit margin is adequate for the profitability of any manufacturing enterprise, AIDAN said.
Signed
(Convenor, AIDAN), others
Address for Communication:
To,
Sri. Ramvilas Pas wan
Honourable Minister of Chemicals and Fertilizers
Shastri Bhawan, Dr. Rajendra Prasad Road
New Delhi 110 001
Tel: 23386519, Fax: 23384020
Sub: Bringing essential drugs under price control - A move in the right direction; Yet some
concerns
Dear Sir,
We are writing this letter to congratulate you on your efforts to bring essential drugs under price
control. This will go a long way in promoting people’s access to drugs. Community Health Cell
(CHC) is a technical resource group in health. We have been involved in community health,
public health and health policy issues for the past twenty-two years. An important part of our
work is towards promoting the use of rational drugs, and working towards ensuring accessibility
and affordability of drugs for all, as a part of the ‘health for all’ movement. Promoting
community health based on the social paradigm, through policy action, training, mainstreaming,
networking and the people’s health movement is CHC’s core thrust.
One of oin long standing complaints was that some of the very good recommendations of the
Report of the Committee on Drugs and Pharmaceutical Industry (popularly known as Hathi
Committee), set up by the Ministry of Chemicals was being neglected. With regard to the issue of
price control, the Report had clearly stated that ‘
there is no justification for the drug industry
charging prices and having a production pattern which is not based upon the needs of the
community but on aggressive marketing techniques and created demand: As you know,
successive governments had brought down the number of drugs under price control from 347 in
1977 to around 25 or so in 2002, by ignoring the important recommendations of the Hathi
Committee. We are very glad that your Ministry has taken this bold step forward.
V
We still have a few concerns which we hope will be taken up in this drug policy itself.
1) The first is that, past experience has shown that when there is price regulation, the
companies switch to production and promotion of drugs which are often irrational or
higher priced alternatives which are outside the list. The government should pre-empt this
by bringing all alternative drugs also under price control, if not, put them under a scheme
of price monitoring.
2) The policy should put in place firm guidelines on conduct of clinical trials in India and
limit new drug approvals to entities which clearly confer a therapeutic and cost advantage.
3) The policy should mandate the removal of irrational formulations which comprise a
major part of the market, ban hazardous drugs, and ensure quality in manufacturing and
testing of drugs.
4) The policy should evolve stricter codes on pharmaceutical promotion, mandate provision
of unbiased prescribing information, implement nationwide pooled procurement schemes
on the lines of Tamil Nadu and Delhi, and improve availability of drugs in the public health
system.
In a country where over 60% of the population lack regular access to essential medicines, the
move to bring essential drugs under the price control is certainly a move in the right direction.
However if we need to reach the estimated 649 million people in India who still lack regular
access to essential medicines [(World Medicines Situation Report, 2004 - (WHO)], there was an
urgent need to institute a comprehensive, balanced and rational drug policy in the country. We
hope your ministry will take up this matter at the earliest. We are willing to provide any sort of
information or support you may need in undertaking this task. Congrats once again!
Copies to:
Mr. Gurdial Singh Sandhu
pXche™k(PharmaCeUtlCalS lndUStry) & Chief Vl9ilance officer' Department of Chemicals &
rcu uui ici I ncaiS
Mrs. Satwant Reddy
Secretary, Department of Chemicals & Petrochemicals
Ministry of Chemicals and Fertilizers
To,
Sri. Ramvilas Paswan
Honourable Minister of Chemicals and Fertilizers
Shastri Bhawan, Dr. Rajendra Prasad Road
New Delhi 110 001
Tel: 23386519, Fax: 23384020
Sub: Bringing essential drugs under price control - A positive move; yet some concerns
Dear Sri. Ramvilas Paswan,
We, at Community Health Cell (CHC) are writing this letter to congratulate you on your
ministry’s efforts to bring essential drugs under price control. This will go a long way in
promoting people’s access to drugs. CHC is a technical resource group working in the public
health domain. We have been involved in community health, public health and health policy
issues for the past 22 years. An important part of our work which is geared towards ‘health for
all’ is to promote the use of rational drugs, and work towards ensuring accessibility and
affordability of drugs for all. CHC’s main goal is to promote community health based on the
social paradigm through policy action, training, mainstreaming, networking and the people’s
health movement.
One of our long-standing issues was that some of the very good recommendations of the Report
of the Committee on Drugs and Pharmaceutical Industry (popularly known as Hathi Committee),
set up by the Ministry of Chemicals, were being neglected. With regard to the issue of price
control, the Report had clearly stated that ‘
there is no justification for the drug industry
charging prices and having a production pattern which is not based upon the needs of the
community but on aggressive marketing techniques and created demand: As you know,
successive governments had brought down the number of drugs under price control from 347 in
1977 to around 25 or so in 2002, ignoring the important recommendations of the Hathi
Committee. We are very glad that the Ministry of Chemicals and Fertilisers has taken this bold
step forward.
We still have a few concerns which we hope will be taken up in this drug policy itself.
1) Past expenenoe suggests that In the light of price regulation, companies ,witch t„ pr„duction
and promotion of alternative, ina.ionally priced drugs outside the control list. The government
srould pre-empt tins by bringing.il alternative drugs also under price control. If this is no, found
feasible, they should at least be brought under a price monitoring scheme.
2) The policy shouid put in pIace firm guidelines on the conduct of clinical trials in India and
limit new drug approvals to entities that clearly confer a therapeutic and cost advantage.
3) The policy should m«„d«te the remov.l of itratlon.l f„™u|„ions a,,, „mpr|se , mjor
of the market, ban hazardous drugs, and ensure quality i„ the mmruf^riug and testing of drugs.
) The pohcy should evolve stricter codes on pharmaceutical promotion; mandate provision of
unbiased prescribing information; implement nationwide, pooled procurement schemes on the
■nes of Tam.1 Nadu and Delhi; and improve availability of drugs in the public health system.
•n a country where over 60»/„ of the population lack regular access to essential medicines, the
move to brmg essential drugs under price control is certainly a move in the right direction
However .f we need to reach the estimated 649 miilion peopie in India who still lack regular
access to essential medicines (World Medicines Situation Report, 2004; WHO), there is an
urgent need to institute a comprehensive, balanced and rational drug policy in the country.
We hope your ministty will take up this matter at the eadiest. We are willing to provide any sort
o mformation or support you may need in undertaking this task. Congratulations once again!
Copies to:
Mr. Gurdial Singh Sandhu
& PetSc7emica(|sharmaCeUtiCa'S
& Chief Vigila"- Officer, Department of Chemicals
Mrs. Satwant Reddy
Secretary, Department of Chemicals & Petrochemicals
Ministry of Chemicals and Fertilizers
Drug Price Regulation: Why is it Necessary Srinivasan.
Dr. Anuraq Bhargava, S.
Will the final form of the pharmaceutical policy again neglect the
predicament of patients, the Indian experience of the free market in drugs
and deliver a body blow to public health?
We begin with a fact and a question. According to the WHO’s World Medicines
Situation report of 2004, India has the dubious distinction of being the country
with the largest number (an estimated 649 million) people without regular access
to essential medicines. At present the Government has failed in providing access
to basic healthcare and medicines to the people of India. Does it then have an
obligation to patients who are vulnerable, ignorant and in distress, from being
exploited by an unregulated healthcare and medicines market? If telephone and
mobile tariffs, and insurance premiums can be regulated, surely medicines serve
an even more critical need. Or don’t they?
I.The current proposed policy and the arguments of the industry:
No policy relating to the health of the Indian people arouses as much interest in
the media as the pharmaceutical policy. The current policy, evolved possibly in
response to a directive of the Supreme Court and a stated commitment under the
common minimum programme, seeks to increase the number of drugs under
price regulation, to encompass the national list of essential medicines. The
pharma sector is astir and has been trying to score points in the media, by
planting scenarios of panic and death knell for the industry. Few counterpoints
are being offered to clarify the real issues at stake, hence this piece.
The industry creates innovative arguments each time there is any talk of price
regulation. It talks of closure of units, of increase in number of spurious drugs,
decline in exports, decline in R&D, and barely concealed threats of scarcity of
drugs if price regulation is put in place. These arguments are specious. The
margins that are being suggested as part of this policy do not curb profits but
only profiteering, which is currently rampant. The proposed margin of upto 200%
on the basic cost of manufacture for the drugs that shall be under price controls
healthy enough for the profitability of any trade. It has been a part of the pharma
policy that any drug developed by indigenous R&D, shall be exempt from price
control for a period of upto 15 years. There have been no claimants, to the best
of our knowledge, for this exemption so far.
2.The arguments for price regulation:
The purchase of drugs is a unique situation.
The pharma industry portrays medicines as being like other consumer goods and
patients being like other consumers. In the case of medicines, the choice is
exercise by a doctor, and not by the consumer, who is usually ignorant of their
nature. The need for medicines is often immediate, obligatory, even life-long, and
has life and death implications. For such a critical and essential commodity,
governments all over the world, even in so-called market economies regulate the
prices of all medicines while providing them, paradoxically, as part of a highly
socialised system of healthcare.
The high personal cost of disease and the consequences of deregulation of
drug prices in the past.
On the contrary in India we have one of the most privatised system of healthcare.
83% of healthcare related expenditure is borne by out-of-pocket expenditure
made by people, most often by the poor who fall sick more often. More than two
thirds of this expense in outpatient illnesses is made on purchase of drugs. Only
13% of the chemical entities made in India are presently under price regulation..
The availability of drugs in the public health system is abysmal by the
Government’s own admission. The increase in healthcare costs, of which drug
price deregulation is a major cause has resulted in an increasing number of
people not seeking healthcare at all. Those who do seek healthcare fall into the
trap of medical poverty. Healthcare costs are becoming a significant cause of
rural indebtedness and liquidation of assets across the country, which is a stark
reality for people like us who deliver healthcare and medicines to India’s poor.
The no. of drugs under price regulation fell progressively from 347 in 1977 to 146
in 1986, 74 in 1995 to a projected 25 or so drugs in 2002. The 2002 policy which
would have virtually done away with price regulation, was stalled on the
interventions of the Karnataka High Court, who ruled that this would make
essential and life-saving drugs out of the reach of ordinary people. This case is
subjudice in the Supreme Court, to which in fact we are also a party. In fact each
episode of deregulation has been followed predictably with a dramatic increase in
drug prices. In 1995 for example the price of a preparations for anemia rose by
177% while the price of anti-TB drugs rose by nearly 90%. Such increases can
put medicines out of reach for millions of people.
The anarchy of the free market: the reality of prices of drugs, which are
outside the price-controlled list.
A strong argument for governmental regulation is provided by the reality of prices
of drugs, which are outside the price-controlled list, a situation marked by
anarchy. 2 reputed companies manufacture the same chemical in the same
strength with a retail price difference of even more than 1000%.Aventis charges
Rs.95 for a single tablet of an antibiotic like Levofloxacin 500 mg, while Cipla
charges only Rs. 6.8 for the same tablet. The same drug for diabetes can be
sold for 80 paise and Rs. 5.50. The companies are freely charging upto 6 times
in the case of a drug for hypertension, upto 15 times in the case of a drug for a
psychiatric ailment, upto 18 times the price of a drug for cancer, without any
logic, and what is disturbing, without any intervention from the government.
Worse the Government is seeking to reassure the pharma sector that even if the
new policy is introduced 67% of the market shall still be free and outside price
control, indicating perhaps that the government shall look the other way in case
of overpricing. The true nature of the market and the insensitivity of doctors to
the price of a drug is revealed by the fact the costlier brands often sell the most.
The real cost of manufacture of drugs: Price regulation is fully compatible
with profitability.
The real cost of manufacturing drugs is often a very small fraction of the retail
price. This is revealed by the prices of drugs in competitive tenders, by the trade
margins that companies offer, and by the humungous amounts they can afford to
spend on drug promotion.
Internationally an Indian company created a stir when in 2001 it offered to sell
quality certified anti-HIV drugs at 3% of the price at which American companies
sell them. At home in India, even in quality conscious bulk procurement
processes like in Delhi and Tamil Nadu, the tender rates of drugs are as low as
2-20% of the market rate, which would be unheard of in any other commodity. A
few years ago, a reputed company bid for supply of a medicine for worms,
Albendazole 400 mg tablets at a mere 22 paise per tablet, while its brand sells
for Rs. 11.90 in the market. A drug for hypertension like Atenolol is procured at
12 paise by Delhi State while in the market the same drug is sold for as much as
Rs. 2.50. For other examples on this scale, see drug procurement prices of Tamil
Nadu Government at www.tnmsc.com and of Gujarat Government at
http://gujhealth.gov.in/CMSO_RCInfo.pdf .)
Trade margins in pharmaceuticals can be astronomical. Dr. Reddy’s Nimesulide
is priced at Rs. 2.70 per tablet, while Cipla offers the same to its traders at 10
paise per tablet. An antibiotic injection like Amikacin made by Alembic has a
retail price of Rs. 64, while the retailer can buy it at Rs. 13.50. Numerous other
freebies are given to the trade, which include free drugs, consumer items,
overseas trips and the like. The pharmaceutical sector needs to explain to the
public how it can afford to sell drugs at even 10% of their MRP to wholesalers
and not suffer from loss of profitability and yet complain bitterly whenever the
MRP is sought to be lowered by the government?
According to the ET intelligence group in 2004 the top 50 companies alone
spent Rs. 5340 crores on drug marketing . All this cost is recovered from
patients in the form of high drug prices. Surely doctors in India can do with
perhaps less lavish conferences and lesser number of gifts for the sake of lower
prices to the patients.
3.The need for a comprehensive, balanced and rational drug policy:
It is possible to balance the public good with private profit in a pharmaceutical
policy. Profitability of drug companies can be ensured while protecting the people
of India from overpricing. The government is planning to put all the 354
medicines in the National List of Essential Medicines under price control. Past
experience suggests that in the light of price regulation, the companies switch to
production and promotion of drugs, often irrational or higher priced alternatives
which are outside the list. The government should pre-empt this by bringing all
alternative drugs also at the very least under a scheme of price monitoring.
The policy should put in place firm guidelines on conduct of clinical trials in India,
limit new drug approvals to entities which clearly confer a therapeutic and cost
advantage, remove irrational formulations which comprise a major part of the
market, ban hazardous drugs, ensure quality in manufacturing and testing of
drugs, evolve stricter codes on pharmaceutical promotion, mandate provision of
unbiased prescribing information, implement nationwide pooled procurement
schemes on the lines of Tamil Nadu and Delhi, and improve availability of drugs
in the public health system.
It needs to be clarified that price regulation is a national policy matter and in no
way incompatible with TRIPS. In fact it is even a greater imperative under TRIPS.
The MNCs are once again eyeing the huge market, which India has to offer, and
pressing for all kinds of deregulation. The pharmaceutical sector in India, which
owes its existence and its success to strong Governmental support, is ironically
clamouring for the same.
The Government and its committees are all aware of the fact of the anarchy of
retail prices, the rise in prices after deregulation, the high trade margins, and the
impact of healthcare costs on people. If it still does not act on this knowledge in
the public interest should it not be deemed complicit in the rising graph of
people’s miseries? It remains to be seen whether concerns for rising healthcare
costs, and the distress of the people or the state of the stock markets will engage
the government in its thoughts and be reflected in its actions.
( Dr. Anurag Bhargava is a practising physician in a rural community health
program, and S.Srinivasan is involved in manufacture of drugs at low-cost for
community health programs all over India. Both are members of the All India
Drug Action Network which has been campaigning on drug issues and a rational
drug policy for over 25 years)
BMJ 2006;333:1036 (18 November), dol:10.1136/brnj.39031,665868.DB
News
Indian health activists criticise voluntary price cuts
by drug industry
Ganapati Mudur
1 New Deihi
Some of India's largest drug companies have reduced the prices of 886 drug
formulations, butherfth activists and some doctors have described the move
as a ploy by the drug industry to evade meaningful price control.
The government last week released a list of formulations for which the drug
companies have voluntarily reduced the prices by amounts ranging from
0.26% to 74% below current prices.
Officials st the Indian Ministry of Chemicds have said that the 11 companies
submitted indvidual lists in response to ongoing government initiatives to
work with industry to reduce the retail price of drugs.
The formulrtions cover many classes of drugs, including analgesics,
antibiotics, antihypertensives, antipsychotics, and vitamins. Government
officials have also said that efforts are under way to reduce the prices of more
formulations. Buthealth activists have criticised the list, saying that it creates
a misleading impression that the drug industry has made important
concessions.
Pharmacology experts say that most of the drugs in the list are not prescribed
by doctors but are sold through retail outlets. “These formulations make up a
tiny fraction of total drug sales in India," said Chandra Gulhati. editor of the
Monthly Index ofMedical Specialities in India.
"None of the companies have reduced the prices of ®iy of their top 20
drugs," Dr Gulhati said. Government officials have said that they are studying
2 of 2
the implications of this list, but they concede that it seems to cover only a
small proportion of revenues from drug sales throughout the country.
Industry officials have defended the list, however, saying that the prices of
drugs in India are already among the lowest in the world. "Competition
12/12/2006 1:58 PM
between producers of generic medicines is one of the factors that ensures
that prices remain low," said an official atone of the 11 companies.
The All India Drug Action Network, which represents non-governmental
organisations that campaign for access to essential medicines, has been
urging the government to introduce price controls that are based on the cost
of production of specific drugs.
"The retail prices of some drugs in India are exorbitant, compared with their
cost of production,” said AnuragBhargava, an internal medicine specialist at
a community hospital in central India.
Health activists cite the relatively low costat which the government procures
drugs as evidence of the real cost of production. "It costs us two rupees to
make a tablet of fluconazole and we sell it at 2.50 rupees (£0.03. $0.04.
0.04) per tablet. But other companies sell this drug for 30 rupees or higher,"
said Sourir^an Srinivassn, an official with a company that manufactures
drugs for government health programmes end which is not among the 11 that
submitted the list.
The All India Drug Action Network has said high trade and retail profit
margins and inefficient price regulation by the government have led to
unrffordable medicines. The government’s commission on macroeconomics
and health said last year that the cost of buying medicines in India made up
80% of out of pocket expenses on treatment costs.
A spiritual foray into an ecological-spiritual ashram run by the NGO—the Pip al
tree
»
All India Drug Action Network (AIDAN)
Towards a people oriented, rational, drug policy!
Ref: CHC/ 9.2 / 2006/ 403
10 October 2006
Shri. Ram Vilas Paswan
Hon’ble Minister of Chemicals and Fertilizers
Shastri Bhawan, Dr. Rajendra Prasad Road
New Delhi 110 001
Tel: 011-23386519, Fax: 011-23384020
Dear Shri. Ram Vilas Paswan,
Sub: Concerns about drug price regulation being stalled; 10 questions to the Hon’ble Minister
The All India Drug Action Network (AIDAN) is a national network of organisations who have been
working on pharmaceutical policy issues since the early eighties. AIDAN members had welcomed the
proposal to regulate drug prices announced a few months ago. But now we are concerned about the
Government’s turnaround under pressure from an industry with immense lobbying power, and of
announcing sops to divert public attention from the real issue of price regulation, which is being put
into cold storage.
After a lot of discussion and consultation among civil society members, we have decided to write to you with
our concerns. We would like you to respond to the queries raised in this letter.
1) In an unusual move, the Government for the first time decided to release the Pharma policy document
in installments. Why did it do so? Part-A, which dealt with the issues other than price control, was
released in the public domain for comments in December last year. Part B which was to deal with
issues related to price control, has even 10 months later, not been released in the public domain.. What
accounts for the delay and the secrecy with regard to Part B? A few months ago, again with much
publicity, the Government announced plans to regulate prices of the 354 medicines in the National
List of Essential Medicines. What happened to this proposal at the cabinet level, and why was
this proposal completely sidelined in the October 2 announcements? Can the Government and
the industry justify that a 150-200% margin on post-manufacturing costs as was proposed by
the Government earlier, is not profitable enough? Can the Government now specify how many
medicines shall have their prices regulated? Can the Government deny that its own committee
which toured many countries abroad found price regulation mechanisms in place in all
countries?
2) Despite the deliberations and recommendations of numerous committees in favor of price control,
including the Drug Price Control Review Committee of 1999; the Sandhu Committee of 2004: and the
task force appointed in 2005 which was chaired by Dr. Pronab Sen. and after the Government had
announced plans to increase number of medicines under price control , it has now has made a 14
member committee to look into the issue yet again. Of these 11 members are from the industry', and 3
from the government. This brazen act violates the very basis of public policy making. Why has the
Minister constituted a committee made up almost entirely of industry representatives to
deliberate on price control while ignoring the representatives of consumers and public health
professionals? Can he explain why 11 out of 14 members of this committee are from the
industry? Is it just meant to be a front for the industry lobby?
Page 1 of 3
Addresses for Correspondence:
Mira Shiva. A-60. Hauz Khas. New Delhi. Tel: 011-26855010. 09810582028. Email: miiashiva@yahoo.com
Gopal Dabade. 57, fejaswinagar. Dharwad 580002. Tel: 0836-2461722. 09448862270. Email: drdabadc@gmail.com
i -
All India Drug Action Network (AIDAN)
1
Towards a people oriented, rational, drug policy!
I
3) The Government is putting the much awaited decision to introduce price control into cold storage, and
has introduced an entirely token set of steps. The district level medicine banks based on so-called
charity by pharmaceutical companies, in return perhaps, for getting the spectre of price control off
their backs, is a matter of concern. Why doesn’t the Government talk of improved availability in the
public health system, which does not necessarily require much additional expenses as the successful
examples of pooled procurement in Tamil Nadu and Delhi have shown? Isn’t the idea of companies
donating 0.5% of their turnover, even as medicines continue to be priced at 1000% of their cost
of manufacture a mere eyewash?
4) The new policy puts a cap on the prices of generic-generic medicines and branded-generics which
account for a minor part of the drug market while leaving the prices of branded drugs intact The
crucial issue is the retail prices of branded drugs, not those of generic or the so-called branded
generics. The top 300 brands in the market alone sell for more than Rs. 18,000 crores. Generic
medicines constitute less than 5% of the market. Can the Government and the Minister point out
any commonly sold generic drug for anemia, hypertension, diabetes, cancer, or even
dehydration?
5) We would request the Hon’ble Minister to define in legal terms what is being meant by branded
generics (a complete misnomer if ever there was one). Can he point out any such preparation
which does not have a brand name? Then how can it be a generic? Can he point out which of
these branded generics a part of the top 300 brands? If these drugs are not the ones which are
prescribed, sold and purchased the most, how will controlling their prices, help the consumers
or regulate the industry?
6) The Ministry of Chemicals investigated trade margins in some branded preparations of commonly
used drugs like Nimesulide, Cetrizine, and Omeprazole, more than 2 years ago, and found margins of
over 1000% in each of them. Why has it taken 2 years to regulate them and protect the
consumer’s interest?
7) The government has turned a blind eye to the profiteering in retail prices of branded drugs in the
market. The government has been allowing companies to market medicines for mental illness which
cost more than 15 times the cost of their competitive brands, antibiotics and anti-cancer medicines
which cost more than 10 times, and medicines for diabetes and hypertension which cost more than 5
times the cost of other competitive brands leading to huge windfalls for the companies. Does the
industry or the Government have any credible explanation for this phenomenon in a sector
where the fundamental choice of the product is not decided by the consumer, but by doctors
who are heavily influenced by the companies?
8) The Chairperson of the National Pharmaceutical Pricing Authority has said that even in the case of a
drug which is priced 14 times its competitor, he cannot intervene unless it can be shown that the
annual rise in its price was greater than 20%. Isn’t the government’s claim that it monitors the
prices of the medicines outside price control, and clamps price control wherever the behavior is
abnormal, a big farce?
9) The Government’s own Commission of Macroeconomics and Health has mentioned that drug costs
in India comprise 70% of outpatient treatment costs. India has the highest number of people in the
world who lack access to essential medicines because of poor availability and affordability. Past
experience has clearly shown abnormal increase in drug prices after price regulation was removed.
Over the past 12 years not a single additional drug has been brought under price control, and at
present only 74 out of nearly 750 pharmaceutical substances are under regulation. There are now
Page 2 of3
Addresses for Correspondence:
Mira Shiva. A-60. Hauz Khas, New Delhi. Tel: 011-26855010, 09810582028. Email: mirashiva@vahoo.com
Gopal Dabade, 57, Tejaswinagar. Dharwad 580002. Tel: 0836-2461722, 09448862270. Email: drdabade@gmail.com
2
All India Drug Action Network (AIDAN)
Towards a people oriented, rational, drug policy!
A
antibiotics which cost Rs. 6000 per day of therapy, and anticancer drugs which cost tens of lakhs per
year. Drug and healthcare costs are becoming leading cause of rural indebtedness. Then, why the
undue delay in implementing the control on drug prices?
10) The policy is silent on the issues emerging from TRIPS and what safeguards will be used, and when,
to ensure affordability of drugs. Poor Indians are being converted into guinea pigs for the world in
clinical trials which are being conducted, flouting all norms of consent, ethics and safety. The policy
is silent on the issue of irrational drugs and hazardous drugs in the Indian market, which no selfrespecting drug regulatory authority in the world would approve of. Drug promotion in India is
completely unregulated and a major contributor to the inflation of drug prices. There is lack of norms
or regulation with regard to prescription quality, and lack of regulation over the kind of dispensing
provided by India s chemists. Why is the drug policy silent on these issues which are of crucial
concern to the citizens of this country?
AIDAN calls on you, Mr. Paswan, and your ministry to answer these questions rather that have been
raised by health care professionals and consumer groups. As a national network of organisations who
have been working on pharmaceutical policy issues, we would like to hear from you on this and also
meet with you to discuss these issues.
Looking forward to hearing from you.
Best wishes,
Sincerely,
Dr. Gopal Dabade
Co-convenor, All India Drug Action Network (AIDAN)
Page 3 of 3
Addresses for Correspondence:
Mira Shiva, A-60, Hauz Khas, New Delhi. Tel: 011-26855010. 09810582028. Email: mirashiva^ vahoo.com
Gopal Dabade, 57, Tejaswinagar, Dharwad 580002. Tel: 0836-2461722. 09448862270. Email: drdabadefajgmail.com
Govt 'open' to inputs on drug pricing policy
ENS ECONOMIC BUREAU
Posted online: Tuesday, June 20, 2006 at 0000 hrs
NEW DELHI, JUNE 19:Under intense pressure from pharma industry, upset with the new
pricing policy, the ministry of chemicals and fertilisers today told a group of senior executives
of pharma MNCs that it is "open" to the industry's suggestions even at this juncture.
Of course, the ministry feels that there is "no meeting point" between its intent to impose
cost-based price control on most of the 354 essential drugs and the industry's proposal that
effective price monitoring could supplant controls. However, the ministry is willing to have a
look at any "via-media option" the industry might put forth, sources said.
Such an option could be included in the Cabinet note as an alternative, before it is moved to
the Cabinet, the sources added.
A draft Cabinet note is currently in circulation in the government. In it, the ministry has
proposed easing of bulk drugs costing, exemption from price control for low-cost drugs
including OTC products and vaccines and so on.
Importantly, the draft also prescribes that while the specified single ingredient formulations of
the drugs on the national list of essential medicines would invite price control, drug companies
would be dissuaded from switching over to newer strengths of these drugs. This, the draft
suggests, would be through automatic price control on newer strengths.
Ranjit Sahani of Novartis India and S Kalyanasundaram of GSK were among the MNC officials
who met senior ministry officials on Monday. Their main plea, it is understood, was to step up
monitoring and avoid cost-based price control.
Industry captains reiterated their stand that with intense competition in most therapeutic
segments, market forces would keep prices from spiralling. Drug prices in India are among the
lowest in the world, they said. The industry leaders also offered to earmark certain percentage
of the production of a company for distribution to the low income population at discounted
prices.
The ministry, in the draft note, proposed price ceilings for drug purchases by the government.
The ceilings are 50% of MRP in case of control-free drugs and 65% of the notified ceiling price
in case of controlled drugs.
editor@expressindia.com
http://www.indianexpress.com/story/6846.html
How the idea for the campaign emerged - a reminder
People’s Partnership in Primary Health Care
How to take it forward
Invitation for a discussion
The dialogue that we had with the government on 6th April 2006, which was
organized in view of World Health Day, was a big disappointment to many of us. It
has reminded us of the need for more concerted and sustained effort on our part in
order to bring the desirable change.
We have done the preparatory work well in all the six districts. The PA of the health
minister was communicated of our disappointment with the minister for not turning
up for the meeting. He is open to the idea that some of us meet the minister to discuss
the issues of concern with him. Similar communication was also held with the director
of health services. The director has suggested that we meet the health secretary.
We know that these piece meal meetings are not going to take us anywhere hence
some of us sat with the Raichur group who was serious in addressing the issues on 7th
April at Community Health Cell. The need to meet with many of you for discussion to
take the campaign forward was expressed. It was suggested that we together prepare
an action plan for a year. The following suggestions had emerged in the discussion,
which we thought must share with you.
1. There is a need for creating awareness on the structure and functioning of Primary
Health Centres and raise the issues of peoples accessibility to PHC among the
other organizations in each district. It was suggested that we start with the district
that took part in the April 6th meeting.
2. Identify health issues of concern in each district together and evolve an
appropriate action for addressing them. (It would be helpful if each of you come
with information on this on 25th April.)
3. There is a need to prepare booklet-containing information regarding Primary
Health Centres and its functions. ( Abhay and Sangeeta of Raichur district have
volunteered to do this)
4. One of the suggestions was to organize protest in front of the Primary Health
Centeres against corruption and poor quality of services etc, as it was done in
Dharwad district. There is a need to think about other strategies too.
5. Elicit more ideas for strategizing our efforts from the members in districts and to
agree upon a few strategies for implementing during the year.
6. There is a need to connect all our efforts at district level and to the health ministry
and directorate of health services at the state level. It was also suggested that a
delegation meets with the health minister and submits a memorandum containing
issues of concern to us. Similarly we need to meet the health secretary and the
director of health services for forming a joint monitoring committee in each
---- Original Message----From: " Jan Swasthya Sahyog" <jss ganiyari@rediffmaiLcom >
To* <-navthom@yahoo.co.uk>; <prasanna aid@yahoo.comvement.org>
Sent: Monday, October 02, 2006 10:00 PM
Subject: statement on drug policy
dear naveen,prasanna,
if you think that the statement is OK, can you make a
shorter/modified version suitable for a press release, the agenda
for the december meet looks fine.
looking forward to seeing you in december.
with warm regards,
anurag
> Jan Swasthya Sahyog
> Office:
> 1-4, Parijat Colony
> Nehru Nagar
> Bilaspur- 495 001
> Chhattisgarh
> Phone and fax: 07752-270 966
Health Centre:
Village & P.O.Ganiyari-495 112
District Bilaspur
Chhattisgarh.
Phone: 07753-244819
Position: 519 (14 views)