RF_DR_7_SUDHA.pdf
Media
- extracted text
-
RF_DR_7_SUDHA
1
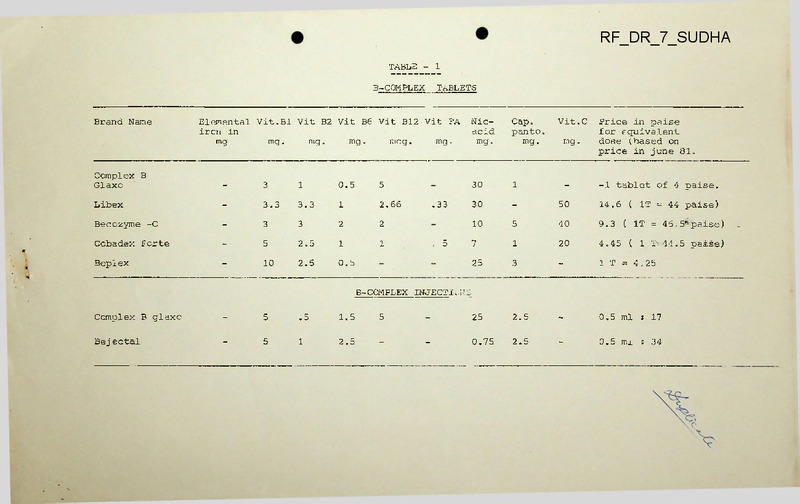
TABLE
TABLETS
B-COMPLEX
Brand Name
Elemental Vit.Bl Vit B2 Vit B6 Vit Bl2 Vit FA
ircn in
mg
mg.
mg .
mcg.
mg.
mg.
Complex B
Glaxo
-
3
1
0.5
5
Libex
-
3.3
3.3
1
2.66
Becozyme -C
-
3
3
2
2
Cobadex Forte
-
5
2.5
1
1
Beplex
-
10
2.5
0.5
.33
. 5
Vit.C
Nicacid
mg.
Cap.
panto.
mg.
30
1
-
-1 tablet of 4 paise.
30
-
50
14.6 ( IT = 44 paise)
10
5
40
9.3
( IT = 45.5
*
paise)
7
1
20
4.45
( 1 T= 44.5 paise)
25
3
-
1 T = 4.25
25
2.5
-
0.5 ml
s 17
0.75
2.5
-
0.5 ml
: 34
mg.
Price in paise
for equivalent
dose (.based on
price in June 81.
B -COMPLEX INJECT!..;'JS
Complex B glaxo
-
5
.5
1.5
5
Bej ectal
-
5
1
2.5
-
-
TABLE - 2
MULTIVITAMIN
Brand name
Vit.Bl
mg.
;vit.B2 ;
’
mg.
i
!
Vit. B6’ Vit.B12 : Vit. i Nic. ! Ca-
mg. ]
mcg
[ FA
,nig.
0.1
TABLETS
; Vit A
; vit.
' Vit. ! Mine- ; iron
J
I.U.
| D IU
J mg.
:
,
! solt
! mg
j
i
C
mg.
i rals
'
500
50
-
4000
400
50
~
5000
3.7
38
! acid j Panto,
;
j mg.
20
5
5000
10
10
i
Vimgran
3
3
1
2
Vitaminets
Forte
10
2
3
1
Multivitqplcx
Forte
2.5
2.5
.5
1.25
.25
25
Price in paise
for equivalent
dose, (based on
prices in June
1981).
13.5 = 1 Tablet
—
18
=1 Tablet
10
(39.5 = IT)
MULTI VITAMIN SYRUP
Multiv.it.7plex
3.5
2.7
Becadex
1.5
1.2
Visyneral
1.5
0.6
ABCDEC
1
Vitamin M
drops
1.5
Alvite
Vi-Syneral
1
5000
1000
50
-
5 ml = 38.65
-
2.5
-
10
-
3000
500
40
-
5 ml = 21.45
1
2
-
10
2
3000
1000
50
5 ml = 32.75
0.4
1
-
5
5000
1000
50
0.6 ml. = 14.2
1.2
.5
-
10
5000
1000
50
Mn K 17.27
Zn
0.6 ml = 17.08
10
2
1
-
-
20
-
5000
500
30
-
55
5
0.5
1.5
-
-
10
-
5000
400
25
-
48
20
MULTI VITAMIN DROPS
2
TABLE NO. 3
IRON WITH MULTIVITAMIN TABLETS
Vit
Folic C
acid
mg
mg.
Cap
Nic
panto. acid
mg
mg
Brand Name
Vit
Bl
mg.
Vit
B2
mg.
Vit
B6
mg.
Vit
B12
mcg.
Iberol
1
1
0.5
4.6
.33
25
1
Exifol
2
2
1
100
5
50
Fesovit
2
2
1
-
50
Fersolate
+ Complex B
Glaxo
3
1
.5
c
-
Elemental
Iron in mg.
Price in paise for equivalent
cose(based on prices in Jane !
5
52.5
6.3(1 tablet costs 19 paise.)
3
10
45
1 T £ 59
-
15
45
1 L = 60.3
1
30
60
1 T = 7.5 paise.
.. .. —
IRON'"WITH ■ FOLIC"' <CID''ASiD 'VIT'B 12
Ferplus
66
5
1
150
66
1 tablet = 34.3
Femitinic
10
1
150
66
1 T = 41.8
Macrafclin
with iron
10
1
-
66
1 T = 6.5
Autrin
9
1. 2
90
70
20.761&1T = 34.6)
(34.6 x 3/5)
Rediplex
10
1
100
68.6
66
21.3 (IT = 32)
(32x 2/3)
3.5
Fersolate
Imforon Fl2
668
3. 2
66.4
154
Uniferon Fl2
66.4
3. 2
66.4
76
(Tables prepared by Nitin Sane,
Pune)
1
TABLE
b-complex
Brand Name
tablets
Elemental Vit.Bl Vit B2 Vit B6 Vit B12 Vit FA
ircii in
mg
mg o
mg o
mg.
mcg.
mg »
Nicacid
mg.
Cap.
panto.
mg.
Vit.C
mg o
Price in paise
for equivalent
dose (.based on
price in June 81
Complex B
Glaxo
-
3
1
0.5
5
-
30
1
-
-1 t<ablet cf 4 paise.
Libex
-
3.3
3.3
1
2.66
.33
30
-
50
14.6
Becozyme -C
-
3
3
2
2
-
10
5
40
9.3
Cobadex Forte
-
5
2.5
1
1
. 5
7
1
20
4.45
Beplex
-
10
2.5
0.5
-
-
25
3
-
1 T == 4.25
25
2.5
-
0.5 ml
i
0.75
2.5
-
0.5 mi
: 34
(
IT = 44 paise)
IT = 46.5 paise)
(
(
1 T--44.5 paise)
B-CCMPLEX INJECTIONS
Complex B glaxo
5
.5
1.5
5
Bejectal
5
1
2.5
-
-
17
1
TABLE
TABLETS
B-COMPLEX
Brand Name
Elemental Vit.Bl Vit B2 Vit B6 Vit B12 Vit FA
iron in
mg.
mg.
mg.
mcg.
mg
mg.
Nicacid
mg.
Cap.
panto.
mg.
Vit.C
mg.
Price in paise
for equivalent
dose (.based on
price in June 81
Complex B
Glaxo
-
3
1
0.5
5
-
30
1
-
Libex
-
3.3
3.3
1
2.66
.33
30
-
50
14.6
-1 t<ablet of 4 paise.
(
IT = 44 paise)
Becozyme -C
-
3
3
2
2
-
10
5
40
9.3
Cobadex Forte
-
5
2.5
1
1
. 5
7
1
20
4.45
Beplex
-
10
2.5
0.5
-
25
3
-
1 T := 4.25
--
25
2.5
-
0.5 ml
s 17
-
0.75
2.5
-
0.5 ml
; 34
IT = 46.5 paise)
(
( 1 T-44.5 paise)
B-COMPLEX INJECTIONS
Complex B glaxo
5
.5
1.5
Bej ectal
5
1
2.5
5
TABLE - 2
MULTIVITAMIN
'Brand name
Vit.Bl
mg.
; Vit.B2 J
‘mg.
J
Vit.Bo; Vit.B12 ! Vit. i Nic . ‘ Ca-
mg.
!
mcg
' aci<i [ Pantc
;
! m<3-
] FA
i mg.
TABLETS
; Vit A
; vit.
' Vit. ]Mine- ; iron
I.U.
! D IU
J mg.
•
,
3
3
1
2
Vitaminets
Forte
10
2
3
1
Multivitaplej
Forte
2.5
2.5
.5
1.25
0.1
.25
‘ solt
; mg
1
' ..
Vimgran
1 rals
c
mg. !
j
J
;
Price in paise
for equivalent
dose, (based on
prices in June
1981).
20
5
5000
500
50
10
10
4000
400
50
-
18
=1 Tablet
25
-
5000
3.7
38
-
10
(39.5 = IT)
5000
1000
■50
—
13.5 = 1 Tablet
MULTI VITAMIN■ SYRUP
Multivitapl :
3.5
2.7
1
Becadex
1.5
1.2
-
2.5
10
-
3000
500
40
Visyneral
1.5
0.6
1
2
10
2
3000
1000
50
-
5 ml = 32.75
20
—
5 m j_
- 38
65
*
5 ml = 21.45
MULTI VITAMIN DROPS
ABCDEC
1
0.4
1
—
5
2
5000
1000
50
—
0.6 ml. = 14.2
Vitamin M
drops
1.5
1.2
.5
-
10
-
5000
1000
50
Mn K 17.27
Zn
0.6 ml = 17.08
Alvite
10
2
1
-
20
-
5000
500
30
-
Vi-Syneral
5
0.5
1.5
-
10
-
5000
400
25
55
-
48
TABLE NG. 3
IRON WITH MULTIVITAMIN TABLETS
Brand Name
Vit
Bl
mg.
Vit
B2
mg.
Vit
B6
mg.
Vit
B12
mcg.
Ibercl
1
1
0.5
4.6
.33
25
1
100
5
50
3
50
FA
Vit
Folic C
acid
mg
mg.
Exifol
2
2
1
Fesovit
2
2
1
-
-
Fersolate
■+ Complex B
Glaxo
3
1
.5
5
-
Cap
Nic
panto. acid
mg
mg
Elemental
Iron in mg.
Price in paise for equivalent
dose(based on prices in June i
5
52.5
6.3(1 tablet costs 19 paise.)
10
45
1 T 4 59
-
15
45
1 L = 60.3
1
30
60
1 T =7.5 paise.
IRON "WITH FOLIC .-.CID
VIT B 12
Ferplus
66
5
1
150
66
1 tablet = 34.3
Femitinic
10
1
150
66
1 T = 41.8
Macrafclin
with iron
10
1
-
66
1 T = 6.5
Autrin
9
1.2
90
70
2O.76161T = 34.6)
(34.6 x 3/5)
Rediplex
10
1
100
68.6
66
21.3 (IT = 32)
(32x 2/3)
3.5
Imforon Fl2
668
3.2
66.4
154
Uniferon F12
66.4
3.2
66.4
76
-
-
-
Fersolate
(Tables prepared by Nitin Sane,
Pune).
COMMUNITY HEALTH CELL
C0M
Mario RO“*
47M«O ’,' ft.c- ■ I O3E - 56° 001
"Operation Antidiarrhoea "
I’-jSD I CO FRIEND CIRCLE
50, LIC Quarters
Rune 411 016
We are sending you herewith the hack-ground paper on
diarrhoea and its treatment.
It is hoped that this paper
will help you in deepening your knowledge about diarrhoea.
The aim is to provide a brief, authentic information which
can be used by doctors, journalists, social workers to educate
the people.
The success of the mass-educational campaign that
is being launched depends largely on to what extent you use
this paper as a back-ground material for educating the laypeople.
Any criticism,
suggestion from you is welcome.
We are thankful to Dr.Raj Anand (Bombay) for his guidance
and Dr. J.Navaranga (Pune) for his valuable comments.
Please lot us know what you have done in "Operation Anti
diarrhoea" to enable us to communicate it to others through
iiFC Bulletin.
If you have already not sent us Rs.5/- towards your contribution to the cost of producing & distributing this paper,
please send this amount if you can to the address given above.
This paper limits itself to acute diarrhoea as a clinical
problem and abstracts from the social-political solution to
the problem of diarrhoea as a social problem.
But even then
we think that it is quite relevant since it would enable us
to oppose irrational approach towards diarrhoea in clinical
practice and the vested interests in the medical world (for
example, drug companies) interested in this irrational
approach.
Sincerely yours,
Anant Phadke
Convener
tiFC
RATxOAALi i<lAi'IAGtSi-xENT 114 ACUTE DIARRHOEA
DR. D.D.JOSHI
—
x.G.student
B.Y.ij.Nair Hospital, Bombay-8
Diarrheea may be defi led as passage of liquid or watery
stools along with increased frequency.
When the patient also passes blood and or mucous with
stools, the condition is known as dysentery.
It must he
stressed that fully breast fed babies who are not being given
any outside hulk food oE water may pass frequent liquid
motions, this is not diarrhoea.
In the first 6 weeks the
stools of such babies may he greenish and contain mucus.
‘These babies remain active, and have normal appetite, they
pass normal amount of urine and continue to gain weight
normally.
The parents of such babies need reassurance and no
drug need he given to such babies.
Acute Diarrhoea remains a major killer and crippier
especially in under 5 age group as evidenced by the following
figures and hence a need to focus on rational and effective
management of actite diarrhoeas.
Unfortunately such a rational
approach is generally lacking.
The figures given below speak
for themselves.
(1)
750 million cases in a year in under 5 age group in Asia,
latin America, and Africa - (a)
(2)
3 to 6 million deaths an jually in these continents in
the same age group - (a)
(3)
case rates from 1 to 12 episodes per child per year-(b)
(4)
In India estimated 1 to 4 million children under age of
5 years die of acute diarrhoea.
The actual mortality figures
may be higher as diarrhoeal diseases may be a contributory
associated factor in death due to other causes. — (c)
Pathogenic Anents in Acute Diarrhoeas
(1)
Rotavirus - (non-medicos may consult the glossay at the
end of this paper for some of the technical words, used) accounts
for upto 50% cases of diarrhoea in age group 6 to 24 months,
visiting treatment facilities.
It also accounts for approxi
mately 10 to 20% of all diarrhoeas in the community.
(2)
Shigella - cause of upto 5% cases of acute diarrhoea
under 5 years of age.
Shigells flexneri is common in the
developing countries.
(3)
Enterotexigenic E.Coli (ETEC) - accounts for upto 25%
of all diarrhoeas in all age groups in the developing countries.
It is one of the common causes of travellers diarrhoea.
(4)
Vibrio Choleras - accounts for only 5 to 10% hospita
lised patients in all age groups in non-epidemic situation.
In cholera endemic areas it is found in children from 2 to
10 years of age.
In newly affected areas initial cases affect
usually adults.
It has emerged as the most important cause of
epidemic diarrhoea due to recent spread of Vibrio Cholerae El
Tor to many countries.-
2
-2-
(5)
Nontyphoid salmonellae - In developing countries upto
10% cases in children.
(6)
Other pathogens - like Vibrio parahaemolyticus, Enteropathogenic E.Coli and Enteroinvasive E.Coli have been isolated
but their relative importance as causes of acute diarrhoea in
developing countries is unknown.
(7)
Protozoa — as a group are not a common cause of acute
dehydrating diarrhoea except Glare'ia lamhlia and Entemoeba
histolytica which are involved in a small percentage of cases.
The relative percentage of each pathogen in causation
of acute diarrhoeas in India is not known.
xhe relative % of
pathogens responsible for acute diarrhoea in a given population
is essential to aval’ misuse of antibiotics.
For example-if
the diarrhoeas in a given population are caused by rotavirus
which causes a self limiting acute diarrhoea, the treatment
must basically be of giving oral rehydration solution.
For
aetiological studies uniform methods of collection, transpor
tation and isolation are must.
MANAGEMENT OF ACUTE DIARRHOEA
I)
Rehydration s
Recent research has revolutionized the management of
acute diarrhoea.
It has been found that in diarrhoea the
absorption of fluids and food is not at fault hut that there
is an active outpouring of chloride, sodium and water into the
intestinal cavity by the injured cells lining the small"
intestine.
This loss of water and electrolytes due to a sort
of leak in the intestine
. (dehydration) is the main dangerous
consequence of diarrhoea.
In majority of the cases, the dia
rrhoea stops on its own without any antibiotic-treatment.
Death due to diarrhoea when it occurs is most of the times due
to dehy -dration.
Hence replacement of fluids and electrolytes
till the body gets rid of the pathogenic organisms becomes
the mainstay of the treatment in acute diarrhoea.
Recent research has now established that replacement of
fluids by mouth is sufficient ih most of the cases. Intra
venous route is needed only in serious cases.
It is necessary
that sodium chloride, should rapidly be absorbed to fully
compensate for their outpouring by the injured cells of the
small intestine.
For this glucose should be added in the
oral rehy-dration S‘'lution (ORS).
Glucose is rapidly absorbed,
and sodium is.automatically absorbed along with glucose. This
"coupling" of sodium with glucose-absorption is taken advan
tage of in oral rehydration.
The fluid prepared for rehy drating a child therefore consists of appropriate quantities
of Glucose and Sodium chloride (ordinary salt).
Addition of
-Potassium Chloride helps to compensate for the loss of Pottassium whereas that of Sodium Bicarbonate counters acidosis.
Correction of acidosis stops vomiting..
The World Health Organization has recommended the ORS
which contains
Nad - 3.5 gms
NaHCog - 2.5 gms
^^1 - 1.5 gms
Glucose- 20 gms in 1 litre of ORS Solution.
..3
-3-
At home ORS can be prepared by taking 1 litre of via ter
and adding 3 level tea-spoons of sugar,^-teaspoon salt and 1/4
teaspoon bicarbonate of Soda.
If no bicarbonate of soda is
available, 1/4 teaspoon of extra salt is added.
Same can also
be made with 4 finger scoops of sugar of jaggery and 3 finger
pinch of salt—(d).
This solution is to he given to the child
in small amounts atatime as much as the child takes. Generally
this fluid should be 1J5 times the amount of stools lost.
ORS
can he given in all conditions except,
1)
Patients with severe.dehydration with signs of shock
2)
In patients who cannot drink due to extreme fatigue,
stupor or coma.
3)
Patients with severe and sustained vomiting.
4)
Paralytic ileus
5)
In patient with severe diarrhoeas where rate of
stool production exceeds absorption of ORs.
In these conditions intravenous therapy has to be used.
/It has been the experience that ORS has brought down the
morbidity and mortality in areas where it is popular.
Also
it causes a weight gain in patients across average episode as
well as over several months time. ' ORS also reduces the inci
dence of dehydration due to insufficient intake. - (e)
There have been various attempts to develop ORS solution with
regional ingredients and including regional staple food-stuffs.
The replacement of glucose (which may not be available) by
sucrose (40 gms) has been useful and effective. - (f)
Attempts have been made to replace ORS with a solution
of- common salt and gur.
In the trial it was found that adult
patients ’with diarrhoea and dehydration are readily r-ehydrated if they drink simple salt-gur solution.
The trial brought
forward, a problem of acidosis which was not corrected in 20%
of patients even after 48 hours.
This failure to correct
acidosis promptly may not be of great clinical significance
in adults with dehydration but more serious consequences of
sustained acidosis may result in children and in severally
affected adults.
Further research on these matters is
awaited. - (g)
The International Centre for Diarrhoeal Disease Research in
Bangladesh conducted a trial in which Sucrose/Glucose was
replaced with rice powder (30 gms).
Rice powder was dissolved
in water and cooked for a few minutes to make a smooth licuid.
In Vitro hydrolysis 80% of the rice powder is converted into
glucose (= 24 gms glucose per litre).
It is stated that as
digestion of rice powder in the intestine liberates the mono
saccharide glucose slowly it does not cause osmotic diarrhoea.
This contrasts favourably against the ORS containing sucrose
or glucose.
These preparations may exacerbate diarrhoea if the
concentration of these sugars exceeds the amount recommended
in ORS.
This finding opens up .the possibility of using a
higher concentration of carbohydrates in ORS which in addition
to providing glucose as the vehicle in transport of electro
lytes, will also provide some energy.
The efficacy of speci
fic enzymes to hydrolyse rice powder remain at a satisfactory
level during diarrhoea due to E.Coli and V.Cholerae.
One more
added advantage in Asia, Latin America and Africa where dia
rrhoea is the main problem, is that, rice is the staple food
in these areas.
4
-4II) Diet :
Maintenance of adequate nutrition is as important as
rehydration in childhood- diarrhoea.
Rehydration prevents
immediate death whereas proper nutrition prevents a possible
downhill course towards death.
Majority of episodes of dia
rrhoea are not severe enough to kill a child on their own. But
they precipitate £ rote in-caloric Malnutrition because of
ignorance, superstitions of parents and even doctors about diet
in diarrhoea.
A child from a poor, rural family would become
weaker and weaker with each episode of diarrhoea and would
finally succumb to even a mild infection.
These slow-killing
diarrhoeas are more frecuent and important than the dramatic
severe episodes; thanks to the traditional practice of starving
the child precisely at a time when it needs adequate feeding
to compensate for the loss of nutrients through diarrhoeal
stools.
Breast feeding should be continued in any event.
In top
fed babies, this is the ideal time to wean12
3the baby away from
the bottle by substituting it with cup and spoon.
The mother
would be in a mood to listen to the advice of discarding the
bottle.
For slightly elder infants, precooked or partially
digested preparations like rice-kanji, or other kanjis, ricedal, sago, curds, butter-milk etc. are very well tolerated.
In a small percentage of cases, milk would have to be withdrawn
because it can not be digested due to a temporary deficiency
of disaccharidase.
But routine withdrawl of milk in diarrhoea
is to be deprecated.
A slight increase in stool-output may occur after feeding
but that should not rrcvant one from feeding the child. Atleast
a part of the food being given will be absorbed and even this
small quantity is important.
HI) Role of Antibiotics :
It is now scientifically established that in majority of
cases of acute diarrhoea, antibiotics have no role to play. As
mentioned above, about half of such episodes are caused by
virus in which of course no antibiotic can help.
Out of the
bacterial diarrhoeas, only some are cut short by antibiotics.
Antibiotics should be used only in the f allowing cases along
with rehydration theropy.
1)
Clear cut evidence of invasive diarrhoeas
stools with high fever).
2)
Suspected Cholera (in cholera endemic areas)
3)
When laboratory investigations are positive
for bacterial infections.
(bloody
A combination of antibiotics and antidiarrhoealsshould
be avoided. Drugs should be used singly and appropriately.
The reasons for cautious, sparing use of antibiotics are
following s1)
Antibiotics are not'useful in majority of cases of
diarrhoea.
2)
Antibiotics are expensive.
3)
Chances of antibiotic resistance development are
present.
.5
-54)
Thera are changes in bowel flora following
antibiotic therapy.
5)
Antibiotics give rise to toxic side effects.
The following antibiotics are useful in various acute
diarrhoea episodes.
In cases of cholera - Tetracyclins shorten the duration
of disease and hence are useful.
Their Dose is 50.®$% per kg
of >>ody weight per day X 3 days.
In case of E'lEC
in this disease with acute episodes of
brief duration, antibiotics are unnecessary.
In case of Shigella - Mild transient diarrhoeas should
not be treated with $rugs.
Only severe bacillary dysentry in
infants with high fe^pr should be treated with Ampicillin
100 mgs per kg per day in 4 divided doses or Trimethoprim Sulfamethoxazole combination twice a day for 5 days.
In case of SaW^nella - Antibiotics do not change the
course of illness in^jAn typhoid salmonella and may actually
prolong the period aufclng which stool culture remains positive
In cases of ■S.ar<;.oaa duo to Giardiasis and Amoebaiasis.
Metronidazole remains ^Jie best‘drug.
The following table
summarizes the rol^ of^antibiotics.
(r)
AOTIfalCtcQBIAL THSRheY
Organism
Selected Antimicrobials
Decreased
duration/
volume of
diarrhoea
Decreased
duration
of positive culture
Escherichia coli
anteropathogenic
ampicillin,
T/s
+
enterotoxigenic
tetracycline,T/S
?+
+
?+
Salmonella spp
chloramphenicol,
0
ampicillin
neomycin,amoxycillin
Shigella spp
T/S,nalidixic acid
++
V.choleras
tetracycline,T/S
++
++
++
++
giardiasis
metronidazole
amebiasis
metronidazole
.
0
++
++
? = controlled studies have not been done in
children
T/S = trimethoprim-sulfamethoxazole
Please note that antibiotics are indicated only in cases
of infection due to one of the four species of organism men
tioned in the table.
Surface Antibiotics - Dike colistin sulphate (e.g.
Walamycin suspension) or soframycin (e.g.Sofrakay) have no
role to play in the management of diarrhoea.
A special word
6
of caution about Neomycin is essential.
(Market preparation
are kaltin — Neomycin, Renokab, Cpmbactin) Neomycin not only
causes renal damage but also makes diarrhoea, dehydration and
nutritional losses ’■r'rs".
It also interferes with ORS absorption - (h)
Chloramphenical alone or in combination should not be •
used since it is not the drug of choice in acute diarrhoea
and can kill patient by its toxicity.
It should be reserved
for-typhoid fever, (market preparation are Chlorostep,Ifistrep,
Enterostep etc.)
IV) Ant id i a r rho ea1s
:
Role of Binding mixtures - Like kaolin and Lectin or
Bismuth and Kaolin (Market preparation are Pectokab, Pecklin,
Linopec, Chlorambin suspension etc.) have no place in manage
ment of acute diarrhoea- (i)
They may solidify the stools.
But the basic pathophysiology is not altered. They tend to
hinder the absorption of antibiotics.
Nothing is gained
beyond a false sense of security.
Solid stools does not mean
that loss of fluids has been stopped.
Role of Antimotility agents - (market preparation are
Lomotil, Imodium, Imosec," Lotnofen, Loperamide)
These drugs
also have no role to play in management of acute diarrhoea.
Diarrhoea protects the body by getting rid of organisms
through increased peristalsis.
These drugs by reducing the
peristalsis hamper this protective function. They merely
create a false sense of security by reducing the frecuency of
passage of stools.
But the outpouring of electrolytes and
water into the intestinal cavity is not arrested.
Lom°.til
has recently come under severe criticism.
It is dangerous
drug because
(1)
It m-sks the fluid loss - (j).
(2)
There is a narrow range between therapeutic and toxic
dose of lomotil - (k)
(3)
There are abnormal sensitivity reactions found in
children - (1)
(4)
It increases the febrile period, of disease and prolongs
excretion of bacteria in stools.
Lomotil is a dangerous combination of drugs-contraindicated for children under age of 2 years and probably never
indicated in childhood diarrhoea.~(m)
Antispasmodics - should be used when colicky pain
accompanies diarrhoea.
;
Cliocuinols
(lodochlorohydroxVouinolins)
The common market preparations are Mexaform and Enterovioform etc.
'There is no evidence to.,suggest that cliocuinols
are effective in prophylaxis of travellers diarrhoea. - (n)
In fact it seems that if large doses of clioquinols can
produce severe and clinically obvious neurological damage;
the accepted smaller dose schedules may cause suhclinical
neurological damage, -(o)
Popular commercial preparations
contain one antimolility^agent besides cliocruinol. This
reduces the frequency of.motions though clioquinols. are not
useful in diarrhoea.
7
-7The com. anies. deny that the neurological damage is common
outside Japan.
This is unconvincing because cliocuinols indu
ced neurological damage has bean observed outside Japan and
identical abnormalities have been produced in animals.
These
dangerous drugs are still available as over the counter drugs
in 109 countries.- (p)
OKS Market Preparations - Orlyte, Orhydrate, Prolyte are
the only ones which come close to the WHO recommended formula.
Others like Emlyte, Lactolyte, Electrol, Electral forte do not
confirm to WHO recommendations and are expensive as well.(s)
'The present task before us includes (1) Finding out %
of cases of acute diarrhoea in India attributable to each
pathogen. (2) Popularising the use of ORE in doctors as well
as lay public.
(3) Making people and doctors aware of the
misuse of antibiotics in non-specific diarrhoea.
REFERENCES
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(1)
(m)
(n)
(o)
(p)
(r)
(s)
WHO/CDD/SER 39.2
Tropical Gastroenterology Vol.l Jan - Mar 1933
Diarrhoeal diseases in infants and. children ICMR-1979
Where there is no Doctor
International Study Group 1931
International centre for Diarrhoeal disease research
Bulletin.
Journal of Tropical Medicine and Hygiene 33(1) 43- 45
Population reoort 1930
WHO/CDD/SER 80.2
Medical Letter 1980
Clinical Paediatrics Jan 1973, 47 - 49.
American Family physician Oct 1976.
Paediatrics 1930
British National Formulary 1931
Paediatrics 1974, pg 339
Lancet 1976 Editorial
Medico-friend circle Bulletin, June 1932
Note on commercial antidiarrhoea1 preparations by
Shirish Data'r presented at the VIII .annual Meeting
of Medico-Friend Circle.
Glossary- For non-medicos
pathogenic agents - disease-causing organisms.
These could he viruses or bacteria or unicellular orga
nisms like amoeba.
Virus - 'These arc the smallest living organisms not
seen by ordinary microscope and arg responsible for a great
variety of illnesses in human beinrs.
As yet scientists have
not been able to invent safe, effective drugs to kill viruses.
Common cold, small-ps>x, chicken-pox, measles, infective
hepatitis (jaundice) mumps, po limyelitis - are caused by
different types of specific viruses.
These diseases are gene
rally self-limiting and antibiotics have no role to paly in
such cases.
About half of the diarrhoeal episodes are caused
by viruses - Rota virus is•the commonest variety involved.
EhiceIla, Salmonella, E.Coli -.etc. are names of diff
erent species of bacteria.
3
1
—3Frotozoa -
Organisms consisting of one cell.
Acidosis in Mood.
excessive concentration of acidic substances
Shock - A serious life-threatening condition characteri
zed by extremely low blood-pressure and great disturbances in
the normal function of the body.
Coma- Life-threatening unconsciouness
Paralytic ilens - Paralysis of normal rhythmic movements
of the intestine (ileum- a part of intestine
In vitro -
In laboratory conditions, outside the human
body
Peristalsis - normal propelling movement of the
intestine consisting of ;fc:fc
*
fc
-£
tf:£
'£
**
ik^
***
****** ■
/*
★
*
*
#**********•£
*
*******★
**
*
*
Five "D"s of Diarrhoea treatment
1) Dehydration - this must be corrected as a top-most
*
*
*
priority
*
2) Diagnosis aetilogical - is important to decide
whether and which antibiotic to be used.
*
*
3) Drugs - appropriate and only when necessary
*
4) Diet - Adequate nutrition is vital to avoid *
downhill
*
*
*
*
’
*
course to death.
5) Disaccharidase deficiency - seen only in a few
patients, but one must keep this possibility in
mind
** * * * * -k * -it * * Jt Jt« * £ * £ ~ * * -Je * -it * *
£
*
* fr * * * * ☆ * * -.Ze x A -A A * A A A A * & •* if * A x * A A A *
-rhythmic contraction and relaxation.
This movement is respon
sible for the slow,forward movement of food.
disaccharidase — an enzyme which breaks down dis
accharides like sucrose (ordinary sugar) into simpler mono
saccharides like glucose.
intraveErous therapy - Treatment by injecting fluids,
medicines in the veins.
binding mixtures - These mixtures are supposed to
"bind" the loose stools, thus rendering them solid.
2intjmotility drugs - 'These drugs reduce the motility
(movement) of the intestine.
Intestinal contents are there
fore retained for a longer time.
Antispasmodics - These drugs relieve the spasm of the
intestine which is responsible for the colicky pain in
diarrhoea.
.9
-9—
L*j>
;J 00000?>=©iJ©©0
AAJ0JJO©J J. ’Jj^->. ©©©©©AA? Abb<
*
J J;b<V .V >? A>
©
©
Only 13 out of 51
Commercial Antidiarrhoaals found useful
©
©
tj
’>
's’
©
©
©
©
©
©
ij
©
Nitin Sane a MFC—subsribar in Pune has studied 51
©
commonly used commercial preparations sold as antidia—
©
rrhoeals to find that only 13 of these contained ingre- ©
dients (in adequate doses) whose efficacy has been scien-Q
tifically .proved.
The rest 33 preparations were found
©
to be useless on account of 'various reasons.
Their
©
breakdown is as follows ©
0
Preparations which -re useless because they contain ©
©
©
©
©
©
©
©
©
©
©
©
©
©
©
©
©
©
©
0
©
©
©
©
drugs in insufficient amount-20 products
irrelevant drugs (for example chloripheniramine
maleate) 9 products.
drugs of doubtful value
(for exnmple-pectin,kaolin) ...25 products
drugs not indicated in diarrhoea
I
(for example-streptomycin ...21 products
drugs which should not be used on account of
their/ toxicity
(for example-diodocuin) ... 11 products
drugs in -wrong combination.
(for example combination of chloramphenicol with
streptomycin) .... 14 products.
©
Many preparations contained more than one of th-e
undesirable features mentioned above.
©
0
©
©
a)
b)
c)
d)
e)
f)
Preparations containing metronidazole, furazolidone
were considered ns useful even if these were accompanied
by ingredients like pectin, kaolin which are of doubtful
value.
The preparations given in the November 1931 issue
of Monthly Index of Medical Specialities (MIMS) under
the heading "antidiarrhoeals" were taken for this study.
MIMS has not included single—ingredient brands of Ampi—
cillin, trmethoprim-sulfnmethoxazole etc. in this list.
The above analysis is based on the latest editions of
Textbooks of Pharmacology by Goodman-Giliman,Martindale
etc.
©
o
0
©
.©
©
0
©
0
©
•.»
©
)
0
0
©
©
©
J J©^M0©?O©0© J J©© 1 .’J© _»jia ? J.AJ0J©JJ >
? ->J© J©0.'- J J© A-A-A0©©©©
Towards rational drug therapy of diarrhea
Mark C. Steinhoff, Vellore.
TABLE I
Anti ;iarrheal Therapy
Type
Ef ficacy
Example
Side Effects
Adsorbents
Kaolin,pectin,
attapulgite, bismuth
salts.
0
Anticholinergics
Atropine,hyoscyamine
0
Opiates
Codeine,tincture of
opium,Lomotil
T
respiratory depre
ssion, coma,
prolongation of
shigellosis
Lactobacillus
Yogurt
0
none
Absorptionincreasing
oral glucose-electrolyte
fluids
+
Hypernatremia
possible
Secretiondecreasinq
(experimental)
Aspirin,
chiorprcmazine
+
salicylate toxicity.
hypotension,
dyskinesia.
adsorption of
antibiotics and
other drugs
.
Salivary,ocular,and
cardiac parasy
mpatholytic effects
TABLE II
ANTIMICROBIAL THERAPY
Organism
Selected Antimicrob
ials.
Campylobacter sp.
Erythromycin
Decreased
duration/
volume of
diarrhea ,
Decreased
duration of
positive
culture.
? "r
? +
Escherichia coli
T/S
+
4-
tetracycline, T/S
*? +
? +
enteropathogenic ampicillin,
enterotoxigenic
Salmonella spp
chloramphenicol,
ampicillin
neomycin ,amoxycillin
0
0
Shigella spp
T/S,nalidixic acid
'++
+'+
V.cholerae
tetracycline,T/S
++
++
giardiasis
metronidazole
++
++
amebiasis
metronidazole
++
? - controlled studies have not been done in children
T/S = trimethoprim-sulfamethoxazole
2
KiBLE III
Recommended Antimicrobial Therapy for specific
Organisms
Shigella species
Trimethoprim - sulfamethoxazole
10 mg T + 50 mg S/kg/day in two doses x 5
day (max.320 mg T + 1.6 gm S/day)
Nalidixic acid 55 mg/kg/day in four doses
x 5 days.
V. choleras
Tetracycline 50 mg/kg/day in four doses
x 3 days
(max 2 gm/day)
boxycycline 6 mg/kg once (max 3 00 mg)
Trimethoprim - sulfamethoxazole
10 mg T + 50 mg S/kg/day in two
doees x 3 days
(max 320 mg T + 1.6 gm S/day)
Giardia lamblia
Metronidazole 15 mg/kg/day in three doses
x 5 days
(max 750 mg/day)
Furazolidine 5 mg/kg/day in four doses
x 7 days
(max 1 gm/day)
Amoebiasis
Metronidazole 40 mg/kg/day in
four doses x 10 days
(max 2.25 gm/day)
Towards rational drug therapy of diarrhea
Mark C. Steinhoff, Vellore.
TABLE I
Anti-diarrheal Therapy
Type
Side Effects
Efficacy
Example
Adsorbents
Kaolin,pec tin,
attapulgite, bismuth
salts.
0
adsorption of
antibiotics and
other drugs
Anticholinergics
Atropine,hyoscyamine
0
Salivary, ocular,and
cardiac parasy
mpatholytic effects
Opiates
Codeine,tincture of
opium,Lomotil
+
respiratory depre
ssion, coma,
prolongation of
shigellosis
Lactobacillus
Yogurt
0
none
Absorptionincreasing
oral glucose-electrolyte
fluids
+
Hypernatremia
possible
Secretiondecreasing
(experimental)
Aspirin,
chlorpromazine
salicylate toxicity
hypotension,
dyskinesia.
TABLE II
ANTIMICROBIAL THERAPY
Organism
Selected Antimicrob
ials .
Campylobacter sp.
Erythromycin
Decreased
duration/
volume of
diarrhea
Decreased
duration of
positive
culture.
Escherichia coli
enteropathogenic ampicillin,
enterotoxigenic
T/S
tetracycline,T/S
Salmonella spp
chloramphenicol,
ampicillin
neomycin ,amoxycillin
Shigella spp
T/S,nalidixic acid
V .choleras
tetracycline, T/S
giardiasis
metronidazole
amebiasis
metronidazole
++
? - controlled studies have not been done in children
T/S = trimethoprim-sulfamethoxazole
. .
2
TABLE III
Recommended Antimicrobial Therapy for specific
Organisms
Shigella species
Trimethoprim - sulfamethoxazole
10 mg T + 50 mg S/kg/day in two doses x 5
day (max.3 20 mg T -I- 1.6 gm S/day)
Nalidixic acid 55 mg/kg/day in four doses
x 5 days.
V.choleras
Tetracycline 50 mg/kg/day in four doses
x 3 days
(max 2 gm/day)
Doxycycline 6 mg/kg once (max 300 mg)
Trimethoprim - sulfamethoxazole
10 mg I t 50 mg S/kg/day in two
doses x 3 days
(max 320 mg T + 1.6 gm S/day)
Giardia lamblia
Metronidazole 15 mg/kg/day in three doses
x 5 days
(max 750 mg/day)
Furasolidine 5 mg/kg/day in four doses
x 7 days
(max 1 gm/day)
Amoebiasis
Metronidazole 40 mg/kg/day in
four doses x 10 days
(max 2.25 Ejm/day)
ToMr. Rajiv Gandhi,
COMMUNITY HEALTH CELL
(Fjrst FloorjSt. Marks Roai
The Prime Minister,
'
lqt,e - 550 001
‘
NEW DELHI.
Dear Mr. Prime Minister
'Ll is learnt that the Government is preparing a new drug policy. As a
doctor, I strongly feel that good quality essential drugs must be made availa
ble to all the people at reasonable prices. I believe that this can be achieved by
a scientific drug policy and hence I suggest the following :
(I) A prioritized essential drug list should be prepared on the
basis of the incidence, severity, prcventability, sequelae of different diseases in
our country. The producrion targets of drugs should be based on this list.
(2) Only those medicines which have been recommended by standard
medical textbooks should be allowed to be sold. A'l other drugs and their
combinations should be banned. A “ Drug Review Committee ’’ should be
set up to make a list of all hazardous drugs or drugs not proved to be useful, or
irrational drug combinations.
(3) Resources currently being wasted on irrational drugs should be
utilized to step up the production of those essential drugs ( like : Vitamin
A, iodized salt, chloroqu n, streptomycin injections, measles and polio-vaccines)
which are currently tn short-supply; and have to be imported in large
quantities.
(4) The quality control mechanism must be radically improved and
stringent punishment must be given to defaulters.
(5) A mechanism independent of the drug companies, must be set
up to supply uptodate information on drugs to doctors. A journal be started
fnr this purpose. Like the Federal Drug Authority (F. D. A.) in the United
State, the information suppled by drug-companies should be scrutinized and
approved by the drug-authorities before being released.
(6) An ethical code for marketing of Pharmaceuticals must be drawn
up and strictly adhered to. Medicines must be sold only under generic name
(with the manufacturer's name in the bracket),
(7) A list of standard over-the-counter (O. T. C.) drugs and their
formulae should be prepared. No other formula should be allowed. The advertisetnent ol O. T. C. drugs sh ruld be precensored to prevent any misleading of
ihe lay-people.
(8) The dire shortage of essential drugs in the Government Sector
especially in the Primary Health Centres should end immediately.
(9) Abolish all taxes on life-saving drugs and control the prices of
all drugs so that drugs are available to the needy at reasonable prices.
( 10) Tne drug-policy should not be decided by the Industry and Che
mical Ministry alone but the Health Ministry should also be equally involved.
(Il) For drugs used in non allopathic systems of medicine, expert bodies
should be formed to prepare a standard formulary and drug-policy for such drugs.
The above principles have to be adopted to make rational drug treat
ment a reality. Apart from these medico-social aspects of the drug-policy, there
are other aspects like self-reliance, which should be decided with the help of ex
perts in the field.
Sir, the Government is planning to enter the ‘ 21st CENTURY ’ with
the help ol modern science and technology. But the approach to problems
also has to be a scientific one. I request you to intervene personally to discard
the existing unscientific approach to the drug-policy issues and to adopt the
scientific principles outlined above. The new drug policy should be prepared
only after a proper public debate.
Thanking you,
Yours faithfully,
Name
Address
Signature
COPMTJNJTY HEALTM CELL
<’7/1,(First FloorjSt. Marks Ho
-J&
BANGALORE - 5u0 001
RATIONALITY STUDY OF
ANTI DIARRHOEAL FORMULATIONS
Dr. SHISHIR J MODAK
RATIONAL DRUG CELL
MEDICO FRIEND CIRCLE
PUBLISHED BY
KERALA SASTRA SAHITHYA PARISHAD
RATIONALITY STUDY OF
ANTI DIARRHOEAL FORMULATIONS
Dr. SH1SHIR J MODAK
RATIONAL DRUG CELL
MEDICO FRIEND CIRCLE
PUBLISHED BY
KERALA SASTRA SAHITHYA PARISHAD
(English)
Rationality Study of Anti Diarrhoeal
Formulations
Written by:
Dr. SHISHIR J. MODAK
Rational Drug cell
Medico Friend Circle
50 LIC Quarters, University Road
Pune 411016.
First Published October 1985
Prinetd and Published by
Kerala Sastra Sahitya Parishad
Trivandrum-695037
Printed at
Prathibha Printers
Trivandrum-695037
Price Rs. two
KSSP
322/85
PAM (eng)
d/8
1/1000
RATIONALITY STUDY OF
ANTIDIARRHOEAL FORMULATIONS
Diarrhoea is the frequent passage of loose stools. Diarr
hoeas are extremely common and endemic in our country.
Almost every child upto the age of 5 years gets 1-2 episodes
of acute diarrhoea in a year. It is a number-one killer in inr
fants and small children. Therefore, every doctor should be
thoroughly trained, regarding proper management of acute
diarrhoeas.
A large number of formulations are sold in the market
as antidiarrhoeal agents. They are usually broad spectrum
and claimed to be effective in diarrhoeas due to different
aetiological factors ranging from ■ bacterial, protozoal,
nonspecific..etc. However, doubts are always raised about
rationality of all these preparations, The purpose of this study
is to assess the rationality and effectivity of multiple
antidiarrhoeal preparations available in the market.
Material and Methods
The 47 different formulations listed under the heading,
'Antidiarrhoeals' in Current Index of Medical Specialities
(CIMS) - May 1984 issue were studied. Each ingredient of
every formulation was evaluated separately on its own merit.
The comments are based on the available scientific literature
on this topic, published in recent standard text books and
periodicals. Finally, each product was graded according to
the resultant rationality of its ingredients.
Antimicrobials as single ingredients (e.g. Ampicillin.
Tetracycline...etc.) are not included in this assessment.
2
RESULTS
Please see the accompanying Table and the resuitant]
gradation of each formulation in the table.
The overal
resultant gradation of each formulation has been done as
follows
A.
Use of the product is justified.
B.
Electrolytes or other irrational ingredients should be
deleted.
C.
The proportion of the ingredients should be altered,
D.
The drug should be avoided and
available strictly against prescription.
E.
it should be
The formulation should be officially banned.
The resultant tally of these formulations was as follows::
ABODE
No. of products :
7 6 9 8 20.
Grade
(Total products studied are 47. Excess number in the
above table is due to some ^products having more than one
grade at a time).
r
Sr. No.
1)
2)
Brand Name
Aristogy! F
(Aristo)
90 ml : Rs. 8.00
Composition
Per 5 ml:
Furazolidone 30 mg
Comments
Grading
Reference
C
13, 14,
12, 18.
2
E
3, 17,
5
•’ Shotgun therapy, incorrect ratio
bet: Fura & Metro. The ratio
should be 1:5.
Pectin - 20 mg
Light Kaolin-1 gm
: Of cosmetic use if at all.
inadequate dose. May actually
increase electrolyte loss.
Chlorambin
suspension
(Anglo-French)
Per 5 ml:
Light Kaolin-1 gm
Pectin-50 mg.
„
60 ml : 6.11
Neomycin - 50 mg.
: Inadequate dose of Neomycin,
Many strains are becoming resi
stant to Neomycin.
Di-iodo-150 mg.
Di-iodo. not a safe drug espe
cially in children. May produce
SMON. Should not be used in
fixed dose combination.
Sr.No.
Brand Name
Composition
Tincture belladona
- 0.06 ml
3)
Chiorostrep
(Cap. & Sus
pension)
(Parke Davis)
Combactin
(CEL Pharma)
60-ml; 5.19
Grading
Per 30 ml:
Neomycin-300 mg
Reference'
: Antimotility drugs should be
avoided in childhood diarrhoea;
should never be added in fixed
dose mixtures.
Per Cap. per 4 ml
:
(Chloramphenicol-125 mg) Chloro-not useful in Salmonella
gastroenteritis; severe side
effects; carrier state may be
prolonged after chloro.
60-ml:Rs. 10.59 Streptomycin
sulphate-125 mg
4)
Comments
Shigella & other enteropatho: genic organisms have become resis
tant to Streptomycin; rapid develop
ment of resistance; sensitization;
should not be combined with
Chloramphenicol for fear of
increased risk of optic neuritis.
E
: Dose of Neomycin inadequate;
Many strains resistant to Neo.
5, 7, 1,
2, 10,12,
3, 5, 12, 17,
Sr. No.
Brand Name
Composition
Comments
Grading
Dicyclomine-10 mg
: Antispasmodic drugs should
not be added in fixed dose mixtures.
Light Kaolin-6 mg
Pectin-130 mg
: As in (1) above
Sod. Lactate-800 mg.
Pot. Chloride-300 mg
Sod. Chloride-470 mg
; Electrolytes should not be
: included in antidiarrhoeal
preparation; inadequate and
wrong proportion.
Reference
See WHO formula
5)
Darzin with
Neomycin
(Chemage)
Per 10 ml:
Light Kaolin-2 gm
Peet in-43 mg
: As in (1) above
60-ml: 6.88
Neomycin-125 mg
: As in (2) above
Sod. Lactate-267 mg
Sod. Chloride-157 mg
Pot. Chloride-100 mg
: As in (4) above
Piptal-4 mg
Antispasmodic drugs should not
be added in fixed-dose mixtures.
E
5,
2 and 12
Sr. No. Brand Name
6)
Dependal Tabs
(Eskaylab)
12 tabs:
7)
8)
9)
2.91
Diarmycin-N
(Nicholas)
60ml: Rs. 5.10
Diarrest
(Ebers)
50ml: Rs. 7.00
Composition
Comments
Per tablet:
Furazolidone-100 mg.
Grading
2,
C
3, 5, 17
Effective antibacterial agent,
also useful in Giardiasis.
May produce SMON; not con
fined to Japan; 7 cases were
reported in Bombay; not a safe
drug; should not be used in
fixed dose combination.
Quinioidochlor-200 mg:
Per 10ml:
Neomycin Sulph. 100mg
Sulphadimidine - 134mg
Pectin-67mg
Light Kaolin-1.34gm
:
As in (1) above
Most of the bacteria are
resistant to sulphas by now.
As in (1) above.
2, 12, 1 6
Per 5ml:
C
Metronidazole - 100mg
Furazolidone - 33mg
Pectin - 75mg
Kaolin - 700mg
References
E
:
12.
D
Dysenchlor Tab Per tab
(S. G. Pharm)
1Otabs: Rs. 1.32 Chloroquinaldol - 100mg :
13, 14, 2
Same as in (1) above.
As in (6) above.
o
2
Sr. No.
10)
11)
Brand Name:
Composition
Emantid
(MM Labs)
60ml: Rs. 6.25
Per 30ml\
Enteromac
(Mac)
Comments
:
Furazolidone - 200mg
Pectin - 1 30mg
hight Kaolin - 6mg
Grading
References
E
2, 12, 3, 7
C
5, 17, 2, 12.
E
3,7
Effective antibacterial agent;
also used in Giardiasis.
As in (1) above
Tincture belladone-0.6ml :
Same as in (2) above.
Sod. Lactate - 800mg
Pot. Chloide - 330mg
Sold. Chloride - 470mg
As in (4) above.
1
Per 5m!
Neomycin - 75mg
:
Same as in (2) above.
Ligt Kaolin - 750mg
Pectin-30mg.
:
See (1) above.
Irrelevant & useless as
64ml: Rs. 4.21
Diphenhydramine-3mg.
.12)
Enterosan
(Wockhardt)
antidiarrihoeal.
Per tab
Berberine HC 1-40mg
May cause hemolytic jaundice.
Di-iodo-300mg
As in (2) above.
10: Rs. 1.86
Homotropine-0.8mg
Sr. No . Brand Name
Composition
13)
Enterostrep
(Dey's)
Per Cap. & per 4ml:
12: Rs. 5.16
60: ml: 6.76
Chloro - 125mg
Strepto - 125 mg
14)
Enterovioform
(Ciba)
500: Rs. 54.00
Per tab ■
15)
Furamide Com
pound (Boots)
Per tab.
10:
Comments
Grading
References
E
Same asin
Chlorostrep
Same as in Chlorostrep
(3) above.
D
Quiniodochlor - 250mg
As in (6) above
B &C
Diloxamide
Furate-250mg
:
Useful in cyst-passers; not
the drug of choice in acute
amoebiasis.
Strepto - 120 mg
:
Shigella &■ other entero
pathogenic organisms have
become resistant to stre
ptomycin; rapid develop
ment of resistance;
sensitisation.
Chloroquine - 50 mg
:
Unnecessary; not indicated
in amoebic dysentry.
Rs. 4.55
15,
5
Sr. No. Brand Name
16)
17)
Composition
Comments
Furamide Susp.
with Neomycin
Per 10 ml.
(Boots)
60 ml: Rs. 5.18
Dilo. Furoate-250 mg.
Not the drug of choice
for amoebiasis.
Neomycin Sulph-80 mg.
Very inadequate dose;
strains becoming
resistant to neomycin.
Furoxone Susp.
(Eskaylab)
:
Grading
References
B & C
3,
Per 5 ml:
Furazolidone-35.7 mg
As in (6) above.
Pectin-75. mg
Light Kaolin-1 gm
As in (1) above.
Loperamide HCI-2 mg
caps.
Antiperistaltic drugs should
not be used in children below
2 yrs. Even in older children
they should be avoided.
5-
A
2, 18.
D
7, 3
57 ml: Rs. 4.90
18)
Imotil
(Cevee Pharma)
4: Rs. 2.75
Sr. No . Brand Name
19)
Kaltin with
Neomycin
(Abbott)
Composition
Comments
Grading
Reference
E
2,18,3,
Per 5 ml.
Kaolin-1 gm
Pectin-22 mg
:
As in (1) above.
Belladona-0.05 ml
Neomycin-50 mg
:
As in (2) above.
Sod. Lactate-133 mg.
Sod. Chlor. 67.2 mg.
Pot. Chlor. - 55 mg.
:
As in (4) above.
7,3,5.
60 ml : Rs. 5.20
20)
Lactisyn
(Griffon)
Per ampoule .
Lactobacillus
lattis - 490 milli.
WHO formula
A
18.
May be useful in infectious
diarrhoeas but results are
not proved by controlled
trials.
:
6 amp: Rs. 12.73
Lactobacillus
acidophillus-490 milli
Streptococcus
thermophillus-10 milli
Streptococcus
Lactis-10 million
Sr. No. Brand Name
21)
Laviest
(Franco-Indian)
12 caps.
Rs. 10.04
22)
23)
Comments
Composition
Grading
Per Capsule:
Dried yeast powder10 million cells
of saccharomyces
Cerevisiae-250 mg.
Linopec
(Pharma
Research)
110 ml.
Rs. 5 40
Per 5 ml:
Lomofen
(Searle)
10 tabs :
Per tab.
Rs. 1.97
Atropine Sulphate
0.025 mg
Furazolidone-50 mg
A
18.
B
2,12,18.
E
3,7.
As (1) above
Light Kaolin-2 gm
Diphenoxylate
HCI-2.5 mg.
Reference
:
Antimotility drugs should not
be used in children below
2 yrs. Even in older children
they should be avoided;
should not be added in
fixed - dose mixture.
:
As (6) above
Sr. No. Brand Name
Composition
24)
Per tablet & per 5 ml:
Lomotil
(Searle)
Comments
Diphenoxylate
10 tabs :
HCI-2.5 mg.
Rs. 1.84
Atropine Sulph-1,025mg.
60 ml : Rs. 6. 59
25)
Grading
Reference
As in (1 8) above.
Per tablet:
Lopamide
(Torrent Labs)
As in (18) above.
Loperamide HCI-2 mg
10 tabs: Rs. 3-00
26)
Per tablet:
Mabino!
Complex (Mac)
Chlorophenoximide-0.2mg.
As in (15) above.
10 : Rs. 4.67
streptomycin 0.16 mg.
lodochlorhydroxyquinoline-0.15 mg.
27)
60 ml : Rs. 8.95
Metronidazole-100 mg
Furazolidone - 35 mg
Kaolin - 1 gm
Pectin - 75 mg
Sr. No. Brand Name
28)
As in (2) above.
Per 5 ml:
Metroquin F Sus
pension (Noel)
Mexaform
(Hind. Ciba Geigy)
Composition
As (1) above.
:
As (1) above.
Comments
E
Quinodochlor - 200 mg
:
As (6) above.
Phanquone - 20 mg
:
Not the drug of choice;
other better drugs available
for amoebiasis.
10 = Rs. 1.80
Oxyphenonium bromide2 mg
29)
Grading
per tab:
3,7
As (23) above.
B, C
Neldar
(Phar-East)
Per 5 ml
60 ml : Rs. 8.18
Neomycin Sulph-50 mg
;
As (2) above
Sulphadimidine-100 mg
:
Most bacteria are now
resistant to sulfas.
:
As in (1) above.
Kaolin-1 gm, Pectin30 mg
Pot. Dihydrogen Phos25 mg
Sod. Lact - 150 mg
Pot. Chlor- 60 mg
Sod. Chlor-100 mg.
Reference
5, 3, 13
12, 18,
WHO formula
As in (4) above.
Sr. No. Brand Name
Composition
30)
Per 30 ml
Dicyclomine HCI-10 mg :
31)
32)
33)
Neo Combactin
(CFL) Pharma)
60 ml: Rs. 5.26
Pectokab
(Chemage)
100 ml :
Rs. 5.98
Pectokab-MF
(Chemage)
Pelopem
(Mercury)
Pot. Chior-330 mg
Sod. Chlor-470 mg.
As (4) above
Per 5 ml
Pectin - 60 mg
Kaolin
1 gm
:
Per 5 ml
Metronidazole - 100 mg :
Furazolidone - 35 mg.
Light Kaolin - 1 gm
Pectin - 75 mg.
Per 15 ml:
Pthalyl Sulphathiazone1 gm
2, 12, 18
C
1. 13, 14
12, 18
D
3, 7
Grading
E
As (2) above
B
Per 6 gm powder
Attapulgite-3
References
12, 18, 3, 7
Most of the bacterial strains
are now resistant.
As (1) above
:
Sod. Chlor-100mg
Sod. Bicarb-81 mg
Pot. Chlor-99mg
Pot. Dihydro Phos-99mg
cal. gluconate-21 mg.
Protoquit
(PFI)
60 ml: Rs. 7.50
B
As (1) above
Comments
Pectin 0.15 gm
Kaolin 3 gm
Tincture opium - 0.08 ml
36)
WHO formula
As (18) above
Composition
Prepared attaPulgite
(Dextromed)
2, 12,
18, 3,
As (1) above
Loperamide HCI - 2 mg :
34)
35)
References
As (4) above
As (1) above
:
Grading
E
Light Kaolin - 600 mg
:
Pectin-130 mg
Neomycin Sulph-300 mg :
Sod. Lact-800 mg
Sr. No. Brand Name
Pesulin-0
(Cadila)
Comments
Limited cosmetic value; does
not decrease fluid loss.
As (4) above.
E
per 5 ml
Furazolidone-50 mg
lodochlorhydroxyquinoline-125 mg
:
Pectin - 75 mg
:
As (6) above
As (2) above
As (1) above
1, 12, 18
Sr. No. Brand Name
38)
Rido!
(Gufic)
Loperamide-2 mg. tab
Salazopyrin
Per tab:
(Carter Wallace)
Salicylazo
50: Rs. 57.35
sulphopyridin 0.5 gm
Per 5 ml :
Salvaol
40)
41)
Saril
(Rallies) (TCE)
D
Sofrakay
(Roussel)
:
WHO
3, 5, 12.
18, 3, 7
formula.
As (1) above.
As (4) above.
Per tab:
Grading
References
E
5, 2, 1
12, 18.
A
5, 12, 18
A
18
As (15) above.
Pthalyl Sulphaphiazole:
200 mg
As (34) above.
Tannic Acid-50 mg.
Not useful
Pectin - 10 mg
As (1) above.
Di-iodo. 125 mg
As (6) above.
Per 5 ml:
Very limited effectlvity
As in (1) abeve.
Per tab:
Lactobacillus 60 million
10: Rs. 4.97
As (2) above.
Comments
Composition
Pectin - 50 mg
Sporlac
(Uni Sankyo)
Effective in ulcerative colitis
E
Soframycin-50 mg
60 ml : Rs. 9.55 Kaolin - 0.5 mg
43)
3,7
A
Streptomycin
Sulfate - 240 mg
42)
E
As (1 8) above.
:
(Associated)
Neomycin Sulph-50 mg :
60 ml : Rs. 7.60 Belladona tincture0.05 mg
Light Kaolin-750 mg
:
Pectin-50 mg
Sod. Lacate - 135 mg
Pot. Chlor-55 mg.
Sod. chlor. 75 mg
Sr. No. Brand Name
References
Grading
Per 4 ml:
Streptomycin base 50 mg: As (15) above
As (2) above
Neomycin base- 25 mg
Pectin 50 mg
Kaolin - 0.75 mg
: As (1) above
Belladona tincture0.05 mg
: As (2) above
As (4) above
Sod Chlor - 25 mg
Pot. Chlor-10 mg
Cal. Lact-10 mg.
Renokab Sus.
(Manners)
39)
Comments
Composition
37)
::
Effectivily not yet proved
by controlled trials.
Sr. No, Brand Name
Composition
44)
Per 5 ml:
Streptomagma
Suspension
(Wyeth)
Comments
Streptomycin Sulfate
50 mg.
Grading
Reference
D
2, 18, 5
12
E
2,
As (15) above.
350 ml: Rs. 17.10i
Kaolin-0.5 gm
Pectin-45 mg
Aluminium
hydroxide - 66 mg
45)
Strepto Paraxin
(Boehringer
Knoll)
10 mg 6 09
As (1) above.
:
It is not an antidiarrhoeal,
of limited use.
:
As (3) above.
Per 5 ml:
Chloro - 125 mg
strepto - 125 mg
5,
7, 10.
E
46)
Streptophenico!
(Mercury)
50 ml : 7.05
47)
Wallamycin
Per 5 ml:
Collistin Sulph-12.5 mg :
(Suspension)
(Carter Wallace)
Kaolin - 438 mg.
Local antibiotic, of
limited use.
30 ml: Rs. 5.67
As (i)above,
Pectin - 33 mg.
A
12, 18
DISCUSSION OF COMMENTS
In the table, the comments are written briefyly against
each ingredient. There is a great amount of repetition as
similar ingredients appear again and again in different formu
lations. Here we would discuss merits and demerits of
different group of drugs.
A)
Antibacterial drugs:
As is now well known, these play little part in the treat
ment of the acute stage of gastroenteritis. Certainly none in
viral gastroenteritis. They may infact do harm by furtherupsetting bowel flora. They can't, in any case, act fast
enough to stop further loss of fluid in a dehydrated child. It
must enough to stop further loss of fluid in adehydrated child.
It must therefore be seriously considered whether they have
any part to to play in the treatment of gastroenteritis5 . If no
pathogens are isolated, there is clearly no point in giving
antibiotics, and it is of interest to note that in 40 to 50%
of cases no organisms can be isolated from stool samples.
Particular mention must be made about some antibiotics
which are inadvertantly used in antidiarrhoeal formulations.
Chloramphenicol
It is a broad spectrum antibiotic effective against several
gram positive and gram negative organisms. However, it is
a potentially toxic drug,. It can produce aplasic anaemia,
other blood dyscrasias, optic neuritis, super-infection., etc.
There is always a danger of development of resistance. There
fore, this drug should be used only in typhoid fever and its
misuse in trivial infections should be stopped at once.
Contrary to expectations, chloramphenicol is not effective in
non-typhoid salmonella gastroenteritis. 5,7 If chloramphenicol
is combined with streptomycin, risk of optic neutitis
increases2 . Therefore,this combination should be condemned.
20
Streptomycin
It is an aminoglycoside antibiotic effective against Myco
bacterium; but also effective against E. Coli, Proteus, H. infludenzae...etc. Formerly, this antibiotic was used in bacillary
agastroenteritis as many organisms have become resistant to
it. 5 Besides there is a danger of rapid development of resisance and sensitisation after oral use.2 The use of this drug
should be reserved for the treatment of Tuberculosis. It
should never be combined with Chloramphenicol as disussed
earlier.
Neomycin
This is a locally acting aminoglycoside antibiotic. It i
effective against some strains of E. Coli. However, organi
sms are fast becoming resistant to this antibiotic. Th
recommended therapeutic dose of neomycin is 100 to 150 mg/
kg/ day. 3 However, almost all the antidiarrhoeal preparations
containing neomycin provide a very inadequate dose of this
antibiotic.
Sulphonamides
Some antidiarrhoeal formulations contain sulphonamide
preparatious. However, effectivity of sulpha preparations has
recently gone down considerably. Most of the organisms
re resistant to them and hence their use is wasteful and
gives rise only to side effects.
Furazolidone
Furazolidone is an anti bacterial agent effective'against a
variety of bacteria. Shigella, Salmonella, E. Coli, Enterococci
are susceptible to it. It is also effective against Giardia. It
is a cheap drug with few side effects. So, it may be widely
used as an antidiarrhoeal drug.
Metronidazole
Metronidazole is the drug of choice in amoebiasis a n
Giardiasis. Therefore, it is commonly found in antidiarrhoeal
21
formulations. Ideally in each case of diarrhoea, stool should
be examined, organisms should be identified and then specific
treatment should be started. However, in our country, where
majority of people cannot afford the cost of stool investigation
and hence, the stool is not examined, the causative organism
is not identified, the combination of metronidazole 4-Fura
zolidone may be justified as broad spectrum antidiarrhoeal.
Aminoquinolines
Quinidochlor or other hydroxyquinoline derivatives are
known to produce Subacute Myelo Optic Neuropathy (SMON)
after prolonged administration. This side effect is not
restricted to Japanese people but several cases have been
reported in Bombay. The exact safe dose and duration of
this drug is not determined especially in children; and, there
fore, this drug should not be used routinely for any nonspecific
diarrhoea. Certainly it should not form part of any fixed doss
antidiarrhoeal mixture.
B)
Antimotility and Antispasmodic Agents:
Lomotil (Diphenoxylate-j-Atropine), Loperamide and
opium derivatives are antiperistaltic drugs. They stop the
loose motions temporarily. They give a false sense of secu
rity without curing the underlying cause. 3 Paralytic ileus,
respiratory depression, cardiac toxicity etc. have been repor
ted in children following ingestion of lomotil. It is not pos
sible to predict the toxic dose in children and while some
may have only the mildest symptoms with relatively large
doses, others develop severe toxicity on ingesting normal
therapeutic dose. Therefore, lomotil should not be used in
children below 2 years; and even in older children these drugs
should be avoided in the presence of infection. These drugs
should be available strictly only against prescription. The
fixed dose formulations containing these drugs may prove
dangerous and should be banned.
Antispasmodic agents like dicyclomine should be used
very carefully to relieve spasmodic pain. They can cause
22
paralytic ileus and should never be included in an antidiarrhoeal fixed dose combination.
As a rule any drug with higher risk of serious toxicity
should not be used in a fixed dose combination, since
in such a combination it is more likely to be used when
not really indicated. Hence, it is recommended that all
such preparations be banned as has been pointed out
above.
C)
Absorbents, Astringents, and binding agents;
Pectin, Kaolin, Bismuth salts are the drugs belonging
to this group. Light Kaolin is a hydrated and purified
aluminium silicate. It is supposed to absorb bacteria and
bacterial toxins. Pectin is purified carbohydrate product
obtained from citrus fruit extracts. It is claimed to form
stools. However, the doss of these drugs provided in
antidiarrhoeal mixtures is too inadequate. Secondly, it is
reported that these drugs may cause loss of electrolytes
by preventing absorption through gastrointestinal tract. These
drugs, if at all, are only of cosmetic value and may actually
mask the severity of the disease.
D)
electrolytes:
In the management of diarrhoeas, administration of
water and electrolytes takes precedence over all other forms
of treatment. However, electrolytes should never be mixed
in antidiarrhoeal drugs. Electrolytes must be administered
with water in proper formula and as per need of indivi
dual patient. Electrolytes provided in the antidiarrhoeal
mixtures are in wrong proportion and too inadequate. They
give rise to false sense of security and may prove harmful
*
CONCLUSIONS
1.
Antibacterial drugs should be used very judiciously
and only if absolutely necessary in the management of
diarrhoea;
2.
All formulations containing combination of chloramphe
nicol and streptomycin should be banned as antidiarrhoeal agents;
3.
All formulations containing streptomycin or chloramp
henicol [alone] should be avoided;
4.
All other antibacterial agents if combined in antidiarrhoeal formulations, should be provided in adequate
dosage, eg. Neomycin, Colistin, Furazolidone, Cotrimexazole...... etc.
5.
Hydroxyquinoline derivatives should not be added in
any of the fixed dose combination. As far as possible
these agents should be avoided and should be available
strictly against prescription.
6.
Antiperistaltic drugs [ lomotil. Loperamide, Opium ]
should not be used in children below 2 years and
when used in children, should be used very cautiously
in proper dosage and for very short period of time.
They should not be added in any fixed dose formu
lations. Antispasmodic drugs like dicyclomine should
be carefully used in children and should never be
added in fixed-dose combinations.
7.
Electrolytes should never be added in fixed-dose
combinations with antidiarrhoeal agents. That gives
false sense of security and may prove harmful.
REFERENCES
13.
Goodman & Gillman, 'The Pharmacological basis of
Therapeutics', 6th edition-1980, Macmillan Publishing
Company.
Satoskar, Kale, Bhandarkar's 'Pharmacology and Pharmacotherapeutics'.
Acute Gastroenteritis, Chapter 7,. P-69 in 'Infectious
diseases of Children' by Saul Krugman & Samual Katz,
7th Edn; The C. T. Mosby Co., 1981.
Current Paediatric Therapy - 10 by Sydney Gells &
Benjamin Kagan-1982 WB Saunders Co.
Infectious diseases, Epidemiolog & Clinical Practice by
A. B, Christie, 3rd Edn-1980, Churchil Livingstone-P
116, 188, 190.
Textbook of Paediatric Infectious Diseases by Feigin
& Cherry, Vol. 1-1983.
'Paediatrics' 17th Edn. by Abraham Rudolph - 1982
(Appleton-Century Crofts), P-1549.
NEJM 262-864-921/1960.
New England Journal of
Medicine
NEJM 256, 1121, 1957.
Valman H. B., Wlman M. J., Use of Antibiotics in
Acute Gastroenteritis among Infants in Hospital BMJ1
1971.
Goetzee M. Leary P. M. — Gentamycin in
E. Coli
Gastroenteritis, Arch. dis. Child. 46, 1971.
Diarrhoeas in children: Indian Journal of Paediatrics
July-Agusut 1980.
Essentials of Paediatrics-by O. P. Ghai/1980.
14.
Textbook of Paediatrics, Vol-2 by Forfar & Arneil, 1984.
1
2.
3.
4.
5.
6.
7.
8.
9.
10,
11.
12.
Report 1966: Annual Report on the work of the Infec
tious disease hospital & their associated laboratory ser
vices, Western regional board hospital, Scot-land, U. K
16.
Davis Joan R„ Farrant W. N„ Uflely Anne: Antibiotic
Resistance of Shigella Sonnei, Lancet, 2:11571970
17.
Ibid, 1968, 479.
18.
AMA drug evaluations: 1984, 5th Edn.
19.
Curtis J A & Goel KM-' Lomotil poisoning in Children' in
Arch of Disease in Child: 54; 1979-p-222.
laren ln
15.
ccr.’wjrjirY t
(First Fl cor)
8Ai<!G.t,u0i>r
G6C 00?
REPORT OF THE PUBLIC BOARD
17 OCTOBER
1984
INQUIRY ON DEPO-PROVERA
FINDINGS CF EACT
I.
DATA AVAILABLE GN THE LONG-TERM RISKS OF DMPA
ARE INSUFFICIENT AND INADEQUATE TO PROVIDE A
BASIS FOR A DECISION WHETHER THE BENEFITS OF
TEE DRUG AS k CONTRACEPTIVE OUTWEIGH ITS DIS
ADVANTAGES UNDER CONDITIONS OF GENERAL MARKET
ING IN THE USA
There are adequate data to assess the efficacy and
benefits of DMPA as a contraceptive,
There is also
sufficient information on its short term side effects and
risks. The drug is clearly a highly offective'contraceptive
with certyin specific advantages, and it does not appear to
pose any immediate irreversible serious side effects.
However, the facts relating to the long terra consequences of
the use cf the drug are inadequate and insufficient to
provide a basis for risk assessment. This is a serious
deficiency in1 light of the specific questions that have been
raised that the drug may have major adverse effects following
its. long terra use or that may become evident only after a
latent period. Most important among those has been the
concern over the drug's carcinogenic potential.
The long term consequences of, he use of DMPA on
neoplasias, in particular of the breast and uterus, as well
as osteoporosis and atherosclerosis are of particular rele
vance for any risk/benefit assessment of the drug's use in
the United States because of the susceptibility cf the
population in this countijy to these diseases.
In the absence of adequate data on the long term
consequences of the drug it is not possible to arrive at any
scientifically defensible conclusion whether or not thebenefits of the drug, when used as a contraceptive, outweigh
its risks..for the average healthy individual in the United
States. ^t also makes it impossible to compare the risk/
benefit ratio . of DMPA with that of other drugs available
for contraception.
II.
DATA FROM THE STUDIES CF RHESUS MONKEYS AND BEAGLE
DOGS CAN NOT BE DISMISSED AS IRRELEVANT TO THE HUMAN
WITHOUT CONCLUSIVE EVIDENCE TC THE CONTRARY.
SUCH
EVIDENCE IS NOT AVAILABLE AT THIS TIME. THEREFORE, THE
FACT THAT MALIGNANT NEOPLASM.S DEVELOPED IN TWO
SPECIES IN TARGET ORGANS CF SEX STEROIDS MUST BE
CONSIDERED AS AN INDICATION OF A POTENTIAL OF
PROGESTOGENS, INCLUDING DM?A, TC PROMOTE THE DEVELOP
MENT CF MALIGNANCIES IN TARGET ORGANS
The findings from animal tests implicate DMPA as a
potential promoter of neoplasias because;
1)
Chronic administration of DMPA was associated with the
development of malignant neoplasias in two mammalian species.
9.
Data are also inadequate to establish effect of MPA on
bone and on the profile of plasma lipoproteins, inform
ation needed to evaluate whether the long term use of
the drug will or will not predispose the individual to
osteoporosis or to atherosclerosis. Cur conclusions of
Law do not rely on this finding.
2
2)
Tho neoplasias developed. in target organs of sex
steroids .
There is good evidence to support the conclusion that
3)
in both species the malignancies were drug related.
4)
There is no evidence to support the conclusion that
the effect of the drug is to be attributed only to tho
administration of excessively high doses and that the effect
of lower doses would differ qualitatively from those of
higher doses,
Therefore, DMPA in these experiments exhibited the
characteristics of a potential carcinogen according to
generally accepted criteria. Under the circumstances, to
dismiss the findings as irrelevant ■ to the human would require
conclusive experimental evidence of fundamental differences
among the species in the basic mechanisms of action of the
hormone or in the responses of target cells; There is as
yet no such evidence at hand. Specifically, there are no
data on the histogenesis of the neoplasias nor on the
mechanism of action of progostogens on the presumed cells of
origin of tho neoplasias in the teat animals. Therefore/
there is no evidence to support the claim that the
malignancies developed either in c ell types unique to the
species or as a result of a species specific response of
target cells to progostogens.
Conversely, data on women who
have been exposed for prolonged periods to the relatively
unopposed action of progostogens are inadequate to warrant
the conclusion that their response to this hormonal state in
terms of neoplasias would differ in some fundamental way
from the two species of test animals.
III.
THS DATA CM THE HUMAN ARE III SUFFICIENT AI-1D INADEQUATE
TO EITHER CONFIRM OR REFUTE THE IMPLICATION OF-THE
ANIMAL DATA THAT DMPA MAE INCREASE THE RISK OF CANCER
IN WOMEN USING DMPA AS A CONTRACEPTIVE.
The available data on the human can not provide a basis
for conclusing whether DMPA, used us a contraceptive, does
or does not influence the incidence of carcinomas in general
or of the accessory organs of reproduction in particular,
because;
)
4.
They fail to provide information'on an adequate number
of long term users of DMPA, or on ex-users who have been
followed for a long enough period of time. There are only
minimal data on subjects that have
used DMPA for 5
years or longer w.i th most of the data reported having boon
obtained from women who have used the drug for 2 years or less
2)
In tho majority of the studios there were no controls
followed in parallel with those using DMPA. In many
studies from developing countries there is not even informat
ion on tho background incidence of the diseases being
studied in DMPA users that could serve as a basis for
comparison.
3)
In a number of tho retrospective studies there is
reason to question the adequacy of the record keeping on
subjects receiving DMPA and, therefore, of the possibility
3
of retrieving the data subsequently for any valid analysis.
To obtain the direct evidence needed tc resolve the
issue would have required purposeful, systematic collection
and recording of data on users of DMPA and appropriate
controls with consideration of the natural history of the
diseases being monitored. Not until recently have subh
studies been initiated. Until they are completed and full
reports of them available their value as evidence is limited.
IV.
IN CADE OF CONTRACEPTIVE FAILURE WITH DMPA, THE RISK
OF A MOTHER GIVING BIRTH TO A. MALFORMED CHILD IS
UNLIKELY TO BE MEASURABLY GREATER THAN THAT POSED BY
THE ORAL CONTRACEPTIVESJ The chaHCE IN EACH CASE CAN
BE ESTIMATED TO BE SMALL ENOUGH NOT TC POSE AN OBSTACLE
TO THE USE OF THE DRUG AS A CONTRACEPTIVE WHEN OTHERWISE
INDICATED.
Data have not been systematically collected on offspring
that have been inadvertently exposed to DMPA in utero.
Conclusions, therefore, can only be based on the body of
epidemiological data obtained on the effects of a variety of
sox steroids, including progostogons, on the developing human
fetus. In these cases, the drugs had boon administered for a
variety of indications and at various times during pregnancy.
This is clearly a less than ideal data base. Nonetheless it
can provide some general estimate of the magnitude of the risk
According to these data the risk of various malformat
ions attributable to protestogens for the various malformat
ions implicated is low. The rate of contraceptive failure
with DMPA when used appropriately is also low. Consequently,
the chance of a mother bearing a malformed child following
contraceptive failure can be estimated to be small. However,
because DMPA is a long acting depot preparation, the
exposure of any susceptible fetus to ihc drug is likely to
be more prolonged than with oral contraceptives.
Consequent
ly, the range of critical and vulnerable events that may come
under the drug's influence may also be expected to be greater
than with oral contraceptives,
.t should bp possible to
counter balance this disadvantages of DMPA by ensuring that
contraceptive failure is kept at a minimum rand taking the
necessary steps to avoid inuecting women already pregnant.
As with oral contraceptives this risk should not, in itself,
constitute a reasonffor not using the drug if otherwise
indicated,
There have been no direct determinations of the concent
rations of MPA in the blood of breast fed infants of
mothers receiving DMPA as a contraceptive nor if tlio amount
of the drug transferred passed onto the infant is sufficient
to have a biological effect. This information is needed
before advocating the use of DMPA as a contraceptive to
lactating mothers in the postnatal period; and before it is
possible to conclude that the drug does not pose any risk
of functional teratogenicity.
4
CONCLUSIOIT OF_ LAW
Accordingly, Upjohn's supplemental now drug application
for Depo-Provera (DIZGPA) sterile- aqueous suspension for
intramuscular injection as a contraceptive agent in humans
does not contain reports of investigation adequate to show
that the drug is safe for use under the conditions
proscribed, fecommended or suggested in the labelling as
required by S 505 (l),
(2) and (4) of the Federal Food,
Drug and Cosmetic Act, and the Information contained in the
supplemental new drug application, combined with other
information about the drug, does not provide sufficient
basis from which FDA can determine thd.t DMPA is safe for
general marketing in to United States.
47/??p^7UIV,'n'
,,'/‘'0-<rS£F!oor)S. .
®AA/GAi.o
'• IL
'“■ - e«o uVi
BAN INJECTABLE CONTRACEPTIVES INDIAN UCMEN DESERVE A BETTER DEAL
A campaign group has been formed in Bombay to protest
against the Drug-controller of India approving NET-BN as a
contraceptive. Two companies - UNICKEM and GERMAN
REMEDIES -- have boon given licences to manufacture this
Today’s protest demonstration in front of Oberoi Towers,
where tho Family planning Association is holding a closed
door conference of ’exports’ on NET-EN. Ue plan to continue
with the campaign and expand it to include all -longacting contraceptives,
That are Injectable Co_ntracoptives_? Injectable Contracept
ives (
* I c) prevent pregnancy more or less in the same way
as oral contraceptives. But they are administered by
in j oction and are long-acting. The best—known ones are
Depo-provera and NET-EN
Depo needs only one injection
every 3 months and nET-EN, one every 2 months.
population control enthusiasts consider in j eatables
the ideal form of contraception for women in the thirdworld because of the ease with which tbox can bo administered
on'a mass -scale and the low failure rate. To those who
look at women in the third world as nothing but faceloss
factors to be considered in any strategy of population
control thex cook up, the benefits seem overwhelming and
the ’risks' in terms of women’s, health negligible. There
has been a concerted campaign lately to ’sell’ the idea .
of I Cs through tho media and elsewhere. The coaforeace
organised by tho Family Planning Asrociation on 28th and. 29th
December
at Gboroi, is part of this ’marketing
strategy’.
Dcpo-proyora and NET-EN -tho controversial ccntracoptives,..
Depo-provera has been the centre of a fight between
Health groups and women's groups on the one side and
Pharmaceutical companies on the other since the sixties,
when tho Upjohn Co. of USA sought approval for it in the
sixties. Upjohn has fought a hard and long battle in the
U 3 unsuccessfully. They desparatoly wanted approval
frofora their exclusive rights on the drug expired. The
campaign in tho U S and elsewhere brought Depo-provera a
'bad name'. Approval for its manufacture has not been given
by the Drug—controller of India. But neither has any
explanation been iven to the public or to interested
groups about why it has not been approved.
Meanwhile,
HETr-EN._ another I C about which not much is known has been
approved in India and licence to manufacture it has been
granted to two companies - Unichon and Gorgan Remedies.
Both Depo-provera and ITET-EN have been used in India
for several years now for research purposes. This research
has been carried out maily on poor women by voluntary
agencies who conduct community health programmes, under the
supervision cf the Indian Council of Medical Research. The
reports of the studies have not been published till today
2
and TCMR haa refused to sake io available co anyone, All
interested parties are supposed to take their word for it
that while Depo is not so good, NET-EN is just fine. Past
experience with contraceptives and other drugs does not
inspire in us any such trust or confidence. We believe
that we have a right to know the details of the research
studies, to make our own investigations and to come to our
own conclusions, We do not consider the masses of women
mere pawns in population control strategies to whom cont
raceptives are 'sold1 on the basis cf incoutivos without .
prior information.
What we do know about ICs is quite disturbing. Upjohn
Go,, conducted two animal safety studies in the sixties a seven year one on. beagle dogs and a ten year one on
monkeys. Within three and a half years of the dog study,
all dogs on high doses and half on low doses were dead due
to. inflamation of uterine lining.
(The two on low doses
who survived had their uteruses removed,) All control
dogs ane survived except one which died of bite wounds
and four which were sacrificed by the researchers. The
dogs also developed cancer of the breasts, drug-induced
diabetes and-various other problems. At this point, Upjohn
declared that beagle dogs were not the ideal animals to
judge risks to hdiman females. Later even the monkey
studies in which cancer of tie uterus occured were said to
be 'irrelevant to human experience'. The history of this
controversy has been marked with disinformation and a
desparate desire on the part of the company to maximise
profits without making sure first that the drug is safe.
Breast cancer, two types of uterine cancer, serious
menttrual disturbancesi and masculinisation of female foetuses
are some of the serious effects of Depo—provera. Others are
depression, decreased libido, nausea, dizziness,(weight
gain without any increase in nutrition)etc,
The W H 0 report on I Cs (1982) says that the
majority of women on I Cs have their menstrual cycle dis
rupted. The extent of disruption is stunning.
"Less than
one third of women on Depo report having any normal
menstrual cycle during the first year of usa&e' and
'approximately half the users ( of NIST-Eli) reported at
least one normal menstrual cyclo during the first year'«
Both the above quotes from the WHO report are examples of
the concerted attempt to underplay the dangers of I Cs, In
fact, a significant number of women stop having their
periods only to haye severe bleeding after injections are
withdrawn while others bleed every day of the. month while
on the drug. But everyone concerned seems to feel that
it is a minor side-effect. For Indian women who hold tho
world record for anemia, it is a very very significant
side effect.
There is far lot
*
information available about NET-ELI
on human metabolism, on. infants exposed to them through
breast-milk or about their carcinogenic properties. No
one seems to know why the majority of women on those
drugs suffer from menstrual chaos. No do they know why
these women put on weight without more nutrition or why
they are depressed.
3
Yet the advocates of I Cs, including the W H C, consider
them an ideal form of contraception, Their favourite
phrase is risk-benefit ratio. According to tz.um if the
benefit outweighs risk, the drug should be used.
BUt the risks arctaken only by women. The benefits are
mainly 1 for the pharmaceutical companies, the population
control experts and the Governments of third world countries-.
There is a lot that is wrong with our family planning
policies. Its always our families and their plans. A
beginm'ng must be made somewhere to correct theip. Lets
start with the newest strategy which is about to be imposed
on the masses of Indian women. Lets struggle against the
inundation of this country with ICS.
OU?; DEMANDS;
;) Ban all long-acting contraceptives and
withdraw approval for NET-EN.
2)
Make public all studies in India an Pepo and NET-PIT.
immediat ‘ely .
3)
Stop experimenting on third world women with hazardous
drugs and contraceptives.
4)
Institute a public enquiry on the controversial
injectable and implanted contraceptives.
join US III OUR STRUGGLE FOR A BUTTER DEAL FOR OUR WOKEN.
Campaign group against long-acting contrqctptives.
1.
Forum Against Oppression Of Women.
"
2)
Women's Centre, Bombay.
3)
Medico-friends' Circle.
4)
' Stroe Mukti Sanghatna
5)
Sangharsh Vahini.
'
Multinationals Sn Indian Drug Industry
—No positive role to play
—Findings of a seminar
I was one of the participants of the Seminar- The Drug Industry and The Indian People-held at Delhi
on 7th and 8th November 1981. It was organized by five different organizations of scientists, medicos. Substan
tial amount of concrete material was presented in this Seminar on how the drug industry, especially the Multina
tionals is deceiving, exploiting the people. In this article, I have tried to present in a somewhat coherent manner,
some of the most important facts presented in different papers in this Seminar. I have also used a couple of
other sources. I bear the responsibility of interpreting the facts and figures. The authors of these papers or the
organizers of this seminar may not agree with my interpretation.
Anant Phadke
Monopolistic Structure
The Pharmaceutical industry all over the commer
cial world is controlled by a few giant carporations.
“ In 1974, the top 30 multinational firms accounted
for 52 percent of the total sale of pharmaceutical
products in the world. In 1973. the top 20 firms
accounted for over 75 per cent of the total ethical
drug sales in the U.S.A, and the U.K. ... Individual
enterprises tend to specialise in sub-markets leading
to a concentration within product classes. For instance
in 1973 according to Roche’s own estimates, their two
main tranquilizer formulations - Librium and Valium
help more than a third of the entire world tranquilizer
market.” (1 )These giant carporations can apply the
latest fruits of scientific, technical research because they
have the resources to do so. They can also set aside
large sums of money to do research for newer, better
drugs. But since their primary motive is to maximise
their profits, this potential is not realised properly.
Instead, their strength is used mainly to manipulate
things to serve their narrow profit-interests. This is
clearly seen if we see their role in a developing country
like India.
The Pharmaceutical industry in India has been
dominated by the giant foreign companies mentioned
above. Even after 30 years of Independence, their
domination continues. In India, “ While the value of
drug production increased from Rs. 10 crores ( almost
solely formulations) in 1948 to Rs. 445 crores in 1973
and Rs. 1376 crores (Rs. 226 crores bulk drugs and
Rs. 1150 crores formulations) in 1979-80, the share
of MNC subsidiaries and minority ventures still rema
ins substantial. In 1973-74, 60 firms with foreign shares
accounted for 70 percent of the country’s total drug
sales. The remaining 30 percent was shared by 116 large
and 2500 small manufacturing companies ” (2) Since
most of the research in Pharmaceuticals in the com
mercial sector of the capitalist world is done by giant
multinationals and since pharmaceutical industry is
protected by patent laws, 90 percent of patents in the
pharmaceutical industry in India are also held by these
foreign controlled companies. What are the ill effects
of this commercial profit oriented, giant monopolistic
sector ? Let us know about these one by one.
(2)
Emphasis on drug formulations
Production of bulk drugs requires setting up
complex manufacturing units here in India. But foreign
companies, which started here as marketing subsidiaries
of their giant parents, are not interested in this. They
have been forced by circumstances into manufacturing
activity. But this mainly consists of importing bulk
drugs from parent companies and merely mixing them
together in various proportions to make various
formulations with particular brand names. In 1978-79
out of a total production of Rs. 220 crores of bulk
drugs in India, the foreign sector accounted for 16 7
per cent, whereas out of Rs. 1050 crores of drug for
mulations, it accounted for 43.8 per cent worth of
formulations.(3) This affinity for formulations exists
because formulations means more profit. The average
profitablity [ pre-tax ] of four foreign companies during
1974-77 was 7 per cent for bulk drugs and 21.8 per
cent for formulations. (4)
One of the main reasons given for allowing the
foreign companies to operate m India is that these
companies will bring their complex technology with
them and thereby help setting up a modern, manufact
uring drug industry in India. Though this has happened
to a certain extent, the main effect has been to thwart
the development of modern drug industry in India.
Since the foreign companies are the prime movers in
the drug industry in India, Indian private companies
also indulge mainly in production of formulations Thus
in 1978-79 these Indian companies accounted for 22.3
per cent of bulk drug production and 32.4 per cent of
formulations. The Public Sector however, produces
14.6 percent of bulk drugs and only 5.7 percent of
d rug-formulations. (°)
Social Waste
As we know, many of the formulations in the market
are useless on account of either unnecessary, wrong
ingredients subtherapeutic dosages, wrong combinations
etc. It is estimated that “ out of Rs 1260 crores
worth of drugs manufactured in our country in 197980, essential and life saving drugs accounted for
Rs. 350 croses only; the rest were pick-ups, Tonics
and formulations of marginal value ” ( 0 )The WHO,
the Indian Medical Association and the Hathi Com
mittee have recommended respectively 200, 156 and
116 essential active substances as essential drugs.
The WHO has in addition recommended 30 comple
mentary drugs for treating rare disorders. To this
list could be added a few rational drug-combinations.
As opposed to these about 250 at the mosti drugs,
in India, about 15000 formulations are being marketed
under different brand names. Most of these are
repetative. An analysis of 289 manufacturing units
(accounting for over 85 percent of drug-production)
showed that in 1972, these units were marketing 244
multivitamin-C preparations, 262 Vitamin B complex
tonics and 126 cough syrrups.(7) Since none of them
are better than any other, the main way of selling
these brands is high-pressure advertising and marketing
This advertising pushes up the price to be paid by the
consumer. “ According to one estimate, as much as 18
per cent of turnover on an average is spent by Phar
maceutical firms on sales promotion in India
”(8)
In case of foreign drug companies, this expense is even
more ... ■ “in the case of 24 foreign drug companies
studied, overhead costs ( including sales promotion
expenditure ) amounted to 33.32 percent during 197477 as oppossed to an average of 20 percent in other
industries.” ( 9 ) These expenses are a huge social waste
They are however necessary for the drug companies
for monopolistic competition amongst themselves.
Irrational combinations
To justify a different brand-name, drug companies
many times add some ingredients to the essential drug.
Most of the times these additions are irrational. A sub
committee under the Drugs Consultative Committee
stated that of the 34 categories of fixed combinations
examined, 23 categories were to be weeded out. We
know many examples of irrational combinations. But
it would not be out of place to quote a couple of
scandalous combinations- “It is well known that
Analgin causes serious blood dycerasias as well as
gastric ulcers. Phenylbutazone and oxyphenbutazone
are equally hazardous drugs. But a combination of
Analgin and phenylbutazone achieves a record sale of
over Rs. 2 crores with:n a year of its introduction...
Amidopyrine is a very toxic drug that is banned the
world over; but most of our antispasmodic combina
tions contain amidopyrine
” In 1979-80 we impor
ted 95 tonnes of Amidopyrine. (T1 ) Because of their
monopoly-control, leading manufacturers can dump
these products into the consumer’s body; doctors
virtually acting as agents of these companies.
Not for the poor
Because of the brand-names, advertising, unnece
ssary ingredients and high profit-margins, most of
these combinations are too costly for the vast majority
of our population. The drugs that the poor need
drugs against tuberculosis, leprosy etc.and also vaccines
are under-produced [see table No. 1 and 2 ] because
those who need these do not have the money to buy
them. Even amongst the Vitamins, a similar pattern is
seen. Vit. B complex preparations of various sorts
(3)
consisting mostly of irrational combinations consumed
by the rich account for 5.5 percent of total drug
production. However Vit—A, the deficiency of which
is extremly widespread ( and turns 12000 children
blind every' year ) amongst the poor; accounts for a
mere 0-3 percent of total drug production. This is
even less than the production of Vit. I< and other
such elements !(14 )
TABLE No. 1 (1 2 1
1978
Name of the
drug
installed capacity
tonnes
Production
tonnes
539
1290
94
558
153
257
176
13
225
45
38
17
1. Isonex
2. PAS and its salts
3. Thiacetazone
4. Streptomycin
5. Chlorbquin
6. DDS and its
derivatives
WHO recommended oral electrolyte powders arc
hardly ever available in the market. The one that is
sold maximum is Electral; but it does not conform to
the WHO formula.
TABLE Nc. 2.(13)
1980-81 (estimates)
Target
Production
lac doses lac doses
Vaccine against
Diphth; Pertu; Tetanus
400
250
210
60
D'phth; Tetanus
Tetanus
Poliomyelitis
145
120
* 70
20
As against this, the drugs consumed mainly by the
well-to-do or pushed by the doctors for their own
interests | for example-lnj. Terramycin ) have been
produced beyond their licensed capacity. Table No. 3
gives figures for Pfizer Ltd.
TABLE No. 3.(15)
Product
INH
PAS and its salts
Terramycin
Protinex
Licensed capacity
metric tonnes.
80
110
14
110
Production
during 1979
metric tonnes
52
94
54 •
290
“ The drug firms, when they find that the profitabi
lity is less, do not use the licenses and letters of intent
granted to them. It was reported that of 32 bulk
items covered by 13 licenses, 21 items were not
produced by Glaxo Laboratories during the last five
years. ”(10)
Very little research for the poor
Out of a total world production of drugs of 50
billion, the developing countries in 1974 imported
about 2.1 billion's worth i.e about 4.2 percent. But
out of an estimated annual research bill of 2 billion,
only 30 million i.e. 1-5 percent was spent by the com
panies on the Tropical Diseases which constitute one
of the most pressing health-problems in developing
countries. This amount is equivalent to the “ cost of
building a few miles of motorway ” says WHO, and
is less than one fiftieth of the annual expenditure on
cancer research. (17) Whatever research that is being
done by Western agencies on the tropical diseases,
takes place in developed countries and is focussed
mainly on the problems which they are concerned with.
For example, The US Walter Reed Army Research
Institute is the only Western agency doing systematic
research in malaria. It got interested in malaria
because of the U.S. involvement in Vietnam where
Malaria caused more American casualties than did the
Vietcong army.1 8
In India, out of 45 foreign companies identified by
the Hathi committee as under the Foreign Exchange
Regulation Act, FERA only 7 companies performed R
and D in the manufacture of oasic drugs. An analysis
of 20 multinationals in India showed that during
1974-1975, the R & D expenditure of these firms
ranged between 15 to 2-5 percent of their sales
turnover. Whereas their parent-companies in the West
spend typically between 5 to 15 percent of their annual
turnover on R & D. (1 9 The Sandoz group as a whole
spends nearly 9 percent of its worldwide turnover on
R & D, whileits Indian subsidiary spent only F4
percent of its turnover on R & D in 1975.(2 °)
The reason for this behaviour is simple. The multi
nationals “ can not afford ” to spend on research on
drugs to be used by the poor; the poor being unable
to pay for the research through higher prices for new
drugs. This state of affairs is not going to change
unless the profit motive of the drug-industry is
abolished, unless human needs take a priority. Drug
research is now no more a virgin ground. Now it costs
around 50 million to develop a new drug, more so in
case of tropical diseases since in this field lot of ground
work needs to be done first. The multinationals are
(4)
not going to change their research strategy unless
strong public pressure forces them to do so.
MNCs not needed in India
Even the fruits of the research done in the Western
countries do not percolate quickly through their
subsidiaries here. This has been shown by a study
made by B. V. Rangarao. [ see table no. 4. ]
TABLE No. 4.21
Name of drug
Sulfadiazine
Sulfathiazole
Tolbutamide
Penicillin-G
Streptomycin
Chloramphenicol
Prednisolone
Year of produc Year of produc
tion abroad
tion in India
1940
1939
1956
1941
1947
1948
1956
1955
1955
1960
1955
1963
1957
1963
Out of 138 drugs listed as major Pharma
ceutical innovations from 1950 to 1967,
only 20
were being manufactured in India in 1973. Now in
the West in spite of growing expenditure on drugsresearch, less and less genuinely new drugs are being
innovated since the scope for newer drugs is becoming
less and less for the developed societies. Thus in 1974,
out of the 1500 drug patents filed in 1972, only 45
(3 percent) were “genuine new drugs, ” 150 (10 per
cent) were “ major modifications, ” and the remaining
1305(87per centjwere purely imitative. 3)This means
that the post-war period of explosion of new, better
drugs is over. Therefoie one of the important argu
ments in favour of allowing the multinationals to
continue here- ‘ they bring new, better drugs ’ - is now
less v lid than ever it was.
As of today, the Indian drug industry is technically
competent to produce most of the drugs that are being
produced here. In 1977, there were 64 foreign contro
lled firms of which only 38 produced bulk drugs num
bering 207. Out of these 207 bulk drugs, 93 were
produced exclusively by the foreign companies. The
Indian sector [ private and public ] was also producing
the rest of these 207 drugs. Out of these 93 drugs,
only 29 were ‘ high technology drugs, ’ the production
of which probably can not be taken up by the Indian
sector because of lack of know-how.(a 3) The Indian
sector has the technology for producing the remaining
64 drugs currently being produced exclusively by the
foreign-sectoj. Amongst the 29 ‘high technology drugs’
some may be closely related chemical analogues. In
that case, this number will go down. Further, ‘ high
technology ’ has become a tricky word. The committee
on High Technology constituted by the Government
has in its report [ October 1979 ] included even the
following in ‘ high technology ’ —use of potentially
explosive materials; use of toxic materials; careful on
line controls
!(24) Even in case of the drugs
which can not be produced by the Indian sector,
Indian companies can enter into technological
collaborations ( as they have been doing currently )
with foreign companies. It is not necessary to allow
foreign companies to operate in India. Thus on
technological grounds it is not at all necessary
to allow foreign companies to operate here.
Pumping off money out of India
MNCs are not only necessary, but they also have
deleterious effects on our Industry and the people.
Some of these have already been outlined above. In
the economic field, we find that MNCs have been
pumping off money out of India, firstly through repa
triation of profits and secondly through “ transfer
pricing. ”
A case study of 42 foreign drug companies showed
that during the period 1968-69 to 1977-78, these
companies repatriated Rs. 45'11 crores out of India
in the form of profits, dividends, royalties, office
expenses etc.(2ri) This means that though these compa
nies earn huge profits by exploiting cheap labour in
India, they do not reinvest all of it here.
Repatriation of profits is only one of the me
chanisms. The other mechanism is “ tranfer pricing. ’’
The subsidiaries of multinationals import raw materials
from parent companies at rates higher than the prices
in the international market This raises the prices of
final products and thereby pumps off money from the
pockets of our people into the coffers of the parent
companies.
A systematic study was made by
Chandrasekhar and Purkayastha to calculate the
amount of money being transferred through this
mechanism. In case of the 29 foreign companies for
which they could get sufficient data, they found that
the outflow through transfer pricing was an estimated
20 to 40 per cent more than the outflow through
repatriations during 1977.
A couple of individual examples will give a concrete
idea about transfer pricing. A forcing subsidiary charged
Rs. 60,000 / Kg. for dexamethasone which was later
reduced to Rs. 16000 / Kg at the intervention of the
Controller of Imports. Gentamycin was being imported
into India by a multinational subsidiary at the rate of
Rs. 45000 / Kg. When import of some drugs was
(5)
canalised through a Government agency, the price
was lowered to Rs. 10,000 / Kg. Similarly the price of
doxycycline was brought down from Rs. 3000 to
Rs. 1500 / Kg.(2')
To S umma rise — Multinationals in Indian drug
industry have hardly any positive role to play. Because
of their profit motive and monopolistic form of
competition of product differentiation through brand
names, advertising etc; these companies are primarily
interested in manufacture of fancy formulations for the
well-to-do at the expense of essential drugs for the
vast majority. For the same reasons, they are not
interested in research in areas vital for our people’s
health Their presence in our country can not be justi
fied on even technological grounds. These companies
have moreover pumped out large sums of money [ out
of the profits gained by exploiting cheap Indian
labour ] into the coffers of their parent company.
In the Delhi Seminar therefore, following resolution
was unanimously approved- “ The multinational drug
companies operating in India should be nationalized.
At the same time, the functioning of the Public Sector
should be improved in order to make it truly national
and truly pro-people. ”
One can not go here into the functioning of the
Indian private sector and the Public Sector. But even
a glance at the functioning of Indian private sector
will show that it is also dominated by monopoly profit
motives. The public Sector has not reversed the basic
trends in the drug industry in India. An analysis of
the drug industry will not of course be complete
without analysing the dynamics of the Indian sector.
All socially concious medicos must know the dynamics
of the Indian Sector also in order to know the impor
tant obstacles before a rational drug policy.
REFERENCES
Unless otherwise quoted, all papers quoted below
2)
C. P. Chandrasekhar and Prabir PurkayasthaMultinational Investment, Profit Repatriation and the
Production of Drugs in India, pp. 129, 130.
3)
Dr. Naresh Banerjee-Common diseases of the
Indian people and their requirements of basic and
bulk drugs; p 12.
4)
Average taken from Chandrasekhar, Purkayastha op. cit. table No. 5.
5)
Dr. Naresh Banerjee op. cit. p. 12.
6)
Banerjee op. cit. p. 12.
7)
Chandrasekhar-Purkayastha; op. cit. p. 14.
8)
Nagesh kumar and Kamal Mitra Chenoy-MNCs
In The Drugs and Pharmaceutical Industry; p. 8.
Chandrsekhar-Purkayastha
9)
op. cit. p. 4.
10)
J. S. Majumdar-Drug Industry-Instruments of
Policy; p. 10.
11 )Dr. W. V. Rane and Dr. A. R. Patwardhan—
Priorities In Drug Manufacture; pp. 6,7.
12)
J. S. Majumdar op. cit. p. 6.
13)
Dr. Ghosh-Pattern of Diseases and The Drug
Production in India, (lecture).
14)
Drs. Rane and Patwardhan, op. cit. Table No. 2.
15)
J. S. Majumdar op. cit; p. 7.
16)
J. S. Majumdar op. cit. p. 8
17)
Anil Agarwal-Drugs and The Third World- an
Earthscan document, London, August. 1978; pp 6, 12,
13.
18)
Anil Agarwal op. cit. p. 16.
19)
J. Manohar Rao-A Note On Research And
Development In Private Pharmaceutical Firms In India
pp. 11.
20)
Nagesh Kumar- Kamal Mitra Chenoy op. cit.
p. 3,8.
21)
B. V. Rangarao-Foreign Technology In Indian
Drug Industry-paper presented at the International
Seminar on Technology Transfer, New Delhi, 1973.
22)
Anil Agarwal, op. cit. p. 25.
were presented at Delhi Seminar. Page numbers are
from rhe yet unpublished cyclostyled papers. The papers
are to be published shortly. Those interested, should
write to-Delhi Science Forum, J-55, Saket, New
23)
J. Manohar Rao, op. cit. 1,2.
24)
Majumdar op. cit. pp. 2,3.
25)
Chandrasekhar- Purkayastha op. cit. p. 5.
Delhi-110017.
26)
Chandrasekhar- Purkayastha op. cit. pp. 7,8.
I)
MNCs in the Indian Drugs and Pharmaceutical
Industry, Nagesh Kumar and Kamal Mitra Chenoy.
pp. 6,7.
27)
Nagesh kumar- Kamal Mitra Chenoy op. cit.
pp. 16, 17. ’
□
Reproduced from Medico-Friend Circle Bulletin; January-February 1982.
Published by Medico-Friend Circle.
Publisher : Anant Phadke, 50, L. I. C. Quarter, Pune-411016.
Printers : Balbodh Mudranalay; 993 Sdashiv peth, Pune-411030.
"Operatioh J»ntidiarrhoea"
iXiEDICO FRIEND CIRCLE
50, LIC Quarters
Rune 411 016
Dear friend,
We are sending you herewith the hack-ground paper on
diarrhoea and its treatment.
It is hoped that this paper
will help you in deepening your knowledge about diarrhoea.
The aim is to provide a brief, authentic information which
can be used by doctors, journalists, social workers to educate
the people.
The success of the mass-educational campaign that
is being launched depends largely on to what extent you use
this paper as a back-ground material for educating the laypeople.
Juiy criticism,
suggestion from you is welcome.
We are thankful to Dr.Raj Anand (Bombay) for his guidance
and Dr. J.Navarange (Pune) for his valuable comments.
ilease lot us know what you have done in "Operation Anti
diarrhoea" to enable us to communicate it to others through
iuFC Bulletin.
If you have already not sent us Rs.5/- towards your con
tribution to the cost of producing & distributing this paper,
please send this amount if you can to the address given above.
This paper limits itself to acute diarrhoea as a clinical
problem and abstracts from the social-political solution to
the problem of diarrhoea as a social problem.
But even then
we think that it is quite relevant since it would enable us
to oppose irrational approach towards diarrhoea in clinical
practice and the vested interests in the medical world (for
example, drug companies) interested in this irrational
approach.
Sincerely yours
Anant Phadke
Convener
R/VTlOR'AL i-iAHAGt&iEHT IM ACUTE DIARRHOEA
DR. D.D.JOSHI
—
R.G.student
S.Y.D.Nair Hospital, Bomhay-3
Diarrhoea may he o.efi led as passage of liquid or watery
stools along with increased, frequency.
When the patient also passes blood and. or mucous with
stools, the condition is known as dysentery.
It must he
stressed, that fully breast fed babies who are not being given
any outside hulk food oE water may pass frecuent liquid
motions, this is not diarrhoea.
In the first 6 weeks the
stools of such babies may be greenish and contain mucus.
These babies remain active, and have normal appetite, they
pass normal amount of urine and continue to gain weight
normally.
The parents of such babies need reassurance and no
drug need he given to such babies.
Acute Diarrhoea remains a major killer and crippier
especially in under 5 age group as evidenced by the following
figures and hence a need to focus on rational and effective
management of acttte diarrhoeas.
Unfortunately such a rational
approach is generally lacking.
The figures given below speak
for themselves.
(1)
750 million cases in a year in under 5 age group in Asia,
latin .America, and Africa - (a)
(2)
3 to 6 million deaths annually in these continents in
the same age group - (a)
(3)
case rates from 1 to 12 episodes per child per year-(b)
(4)
In India estimated 1 to 4 million children under age of
5 years die of acute diarrhoea.
The actual mortality figures
may be higher as diarrhoeal diseases may be a contributory
associated factor in death due to other causes. — (c)
Pathogenic Agents in Acute Diarrhoeas
(1)
Rotavirus - (non-medicos may consult the glossay at the
end of this paper for some of the technical words used) accounts
for upto 50% cases of diarrhoea in age group 6 to 24 months,
visiting treatment facilities.
It also accounts for approxi
mately 10 to 20% of all diarrhoeas in the community.
(2)
Shigella - cause of upto 5% cases of acute diarrhoea
under 5 years of age.
Shigells flexneri is common in the
developing countries.
(3)
Enterotoxigenic E.Coli (ETEC) - accounts for upto 25%
of all diarrhoeas in all age groups in the developing countries.
It is one of the common causes of travellers diarrhoea.
(4)
Vihrio Cholerae - accounts for only 5 to 10% hospita
lised patients in all age groups in non-epidemic situation.
In cholera endemic areas it is found in children from 2 to
10 years of age.
In newly affected areas initial cases affect
usually adults.
It has emerged as the most important cause of
epidemic diarrhoea due to recent spread of Vibrio Cholerae El
Tor to many countries.
2
-2(5)
Nontyphoid salmonellae - In developing countries upto
10% cases in children.
(6)
Other pathogens - like Vibrio parahaemolyticus, Enteropathogenic E.Coli and Enteroinvasive E.Coli have been isolated
hut their relative importance as causes of acute diarrhoea in
developing countries is unknown.
(7)
Protozoa - as a group are not a common cause of acute
dehydrating diarrhoea except Giardia lamhlia and Entemoeba
histolytica which are involved in a small percentage of cases.
The relative percentage of each pathogen in causation
of acute diarrhoeas in India is not known.
x'he relative % of
pathogens responsible for acute diarrhoea in a given population
is essential to- avai misuse of antibiotics.
For example-if
the diarrhoeas in a given population are caused by rotavirus
which causes a self limiting acute diarrhoea, the treatment
must basically be of giving oral rehydration solution.
For
aetiological studies uniform methods of collection, transpor
tation and isolation are must.
ItANAGEi'iENT OF ACUTE DIARRHOEA
I)
Rehydration s
Recent research has revolutionized the management of
acute diarrhoea.
It has been found that in diarrhoea the
absorption of fluids and food is not at fault but that there
is an active outpouring of chloride, sodium and water into the ’
intestinal cavity by the injured cells lining the small
intestine.
This loss of water and electrolytes due to a sort
of leak in the intestine
. (dehydration) is the main dangerous
consequence of diarrhoea.
In majority of the cases, the dia
rrhoea stops on its own without any antibiotic-treatment.
Death due to diarrhoea when it occurs is most of the times due
to dehy -dration.
Hence replacement of fluids and electrolytes
till the body gets rid of the pathogenic organisms becomes
the mainstay of the treatment in acute diarrhoea.
Recent research has now established that replacement of
fluids by mouth is sufficient ih most of the cases. Intra
venous route is needed only in serious cases.
It is necessary
that sodium chloride, should rapidly ba absorbed to fully
compensate for their outpouring by the injured cells of the
small intestine.
For this glucose should be added in the
oral rehy-dration solution (ORS).
Glucose is rapidly absorbed,
and sodium is automatically absorbed along with glucose. This
"coupling" of sodium with glucose-absorption is taken advan
tage of in oral rehydration.
The fluid prepared for.rehy drating a child therefore consists of appropriate quantities
of Glucose and Sodium chloride (ordinary salt).
Addition of
Rotasslum Chloride helps to compensate for the loss of Pottassium whereas that of Sodium Bicarbonate counters acidosis.
Correction of acidosis stops vomiting..
The World Health Organization has recommended the ORS
which contains
Nad - 3.5 gms
NaHCo3 - 2.5 gms
^C1 - 1.5 gms
Glucose- 20 gms in 1 litre of ORS Solution.
3
-3—
At home OHS can be prepared by taking 1 litre of water
and adding 3 level tea-spoons of sugar,^teaspoon salt and 1/4
teaspoon bicarbonate of Soda.
If no bicarbonate of soda is
available, 1/4 teaspoon of extra salt is added.
Same can also
he made with 4 finger scoops of sugar of jaggery and 3 finger
pinch of salt-(d).
This solution is to he given to the child
in small amounts atatime as much as the child takes. Generally
this fluid should he 11$ times the amount of stools lost.
ORS
can be given in all conditions except,
1)
Patients with severe dehydration with signs of shock
2)
In patients who cannot drink due to extreme fatigue,
stupor or coma.
3)
Patients with severe and sustained vomiting.
4)
Paralytic ileus
5)
In patient with severe diarrhoeas where rate of
stool production exceeds absorption of ORS.
In these conditions intravenous therapy has to be used.
It has been the experience that ORS has brought down the
morbidity and mortality in areas where it is popular.
Also
it causes a weight gain in patients across average episode as
well as over several months time. ' ORS also reduces the inci
dence of dehydration due to insufficient intake. - (e)
There have been various attempts to develop ORS solution with
regional ingredients and including regional staple food-stuffs.
The replacement of glucose (which may not be available) by
sucrose (43 gms) has been useful and effective. - (f)
Attempts have been made to replace ORs with a solution
of common salt and gur.
In the trial it was found that adult
patients with diarrhoea and dehydration are readily rehydra
ted if they drink simple salt-gur solution.
The trial brought
forward a problem of acidosis which was not corrected in 20%
of patients even after 48 hours.
This failure to correct
acidosis promptly may not be of great clinical significance
in adults with dehydration but more serious consequences of
sustained acidosis may result in children and in severally
affected adults.
Further research on these matters is
awaited. - - (g)
The International Centre for Diarrhoeal Disease Research in
Bangladesh conducted a trial in which Sucrose/Glucose was
replaced with rice powder (30 gms).
Rice powder was dissolved
in water and cooked for a few minutes to make a smooth licmid.
in Vitro hydrolysis 80% of the rice powder is converted into
glucose (= 24 gms glucose per litre).
It is stated that as
digestion of rice powder in the intestine liberates the mono
saccharide glucose slowly it does not cause osmotic diarrhoea.
This contrasts favourably against the ORS containing sucrose
or glucose.
These preparations may exacerbate diarrhoea if the
concentration of these;sugars exceeds the amount recommended
in ORS.
This finding-opens up'.the possibility of using a
higher concentration of carbohydrates in ORS which in addition
to providing glucose as the vehicle.in transport of electro
lytes, will also provide some energy.
The efficacy of speci
fic enzymes to hydrolyse rice powder remain at a satisfactory
level during diarrhoea due to E.Cbli and V.Oholerae.
One more
added advantage in Asia, Latin America and Africa where dia
rrhoea is the main problem, is that, rice is the staple food
in these areas.
..4
-4-
II) Diet :
Maintenance of adequate nutrition is as important as
rehydration in childhood- diarrhoea.
Rehydration prevents
immediate death whereas proper nutrition prevents a possible
downhill course towards death.
Majority of episodes of dia
rrhoea are not severe enough to kill a child on their own. But
they precipitate £ rotein-caloric Malnutrition because of
ignorance, superstitions of parents and even doctors about diet
in diarrhoea.
A child from a poor, rural family would become
weaker and weaker with each episode of diarrhoea and would
finally succumb to aven a. mild infection.
These slow-killing
diarrhoeas are more frequent and important than the dramatic
severe episodes; thanks to the traditional practice of starving
the child precisely at a time when it need.s adequate feeding
to compensate for the loss of nutrients through diarrhoeal
stools.
Breast feeding should be continued in any event.
In top
fed babies, this is the ideal time to wean the baby away from
the bottle by substituting it with cup and spoon.
The mother
would be in a mood to listen to the advice of discarding the
bottle.
For slightly elder infants, precooked or partially
digested preparations like rice-kanji, or other kanjis, ricedal, sago, curds, butter-milk etc. are very well tolerated.
In a small percentage of eases, milk would have to be withdrawn
because it can not be digested due to a temporary deficiency
of disaccharidase.
But routine withdrawl of milk in diarrhoea
is to be deprecated.
A slight increase in stool-output may occur after feeding
but that should not prevent one from feeding the child. Atleast
a part of the food being given will be absorbed and even this
small quantity is important.
ill) Role of Antibiotics •
It is now scientifically established that in majority of
cases of acute diarrhoea, antibiotics have no role to play. As
mentioned above, about half of such episodes are caused.by
virus in which of course no antibiotic can help.
Out of the
bacterial diarrhoeas, only some are cut short by antibiotics.
Antibiotics should be used only in the following cases along
with rehydration therapy.
1)
Clear cut evidence of invasive diarrhoeas (bloody
stools with high fever).
2)
Suspected Cholera (in cholera endemic areas)
3)
When laboratory investigations are positive
for bacterial infections.
A combination of antibiotics and antidiarrhoealsshould
be avoided. Drugs should be used singly and appropriately.
'The reasons for cautious, sparing use of antibiotics are
following
1)
Antibiotics are not useful in majority of cases of
diarrhoea.
2)
Antibiotics are expensive.
3)
Chances of antibiotic resistance development are
present.
5
-54)
There are changes in bowel flora following
antibiotic therapy.
5)
jAntitiotics give rise to toxic side effects.
The following antibiotics are useful in various acute
diarrhoea episodes.
In cases of cholera — Tetracyclins shorten the duration
of disease and hence are useful.
Their Dose is 50 ®$s, per kg
of >>.ody weight par day X 3 days.
In case of ETEC
in this disease with acute episodes of
brief duration, antibiotics are unnecessary.
In case of Shigella - Mild transient diarrhoeas should
not be treated ’with $rugs.
Only severe bacillary dysentry in
infants with high fosjer should be treated with Ampicillin
100 mgs per kg per day in 4 divided doses or Trimethoprim Sulfamethoxazole combination twice a day for 5 days.
In case of SaJJ^pella - Antibiotics do not change the
course of illness in hpAn typhoid salmonella and may actually
prolong the period aufclng which stool culture remains positive
In cases of djLar.J;.oea due to Giardiasis and Amoebaiasis.
Metronidazole rema/ns- ®ie best .drug.
The f ollowing table
summarizes the rol^ of/antibiotics.
(r)
AOTIhlCrtOBIAi. THiSRhrY
Selected Antimicrobials
Decreased
duration/
volume of
diarrhoea
anteropathogenic
ampicillin, T/s
+
+
enterotoxigenic
tet racycline,T/S
Organism
Decreased
duration
of positi—
ve culture
Escherichia coli
?+
?+
Salmonella spp
chloramphenicol,
0
ampicillin
neomycin, amoxycillin
0
Shigella spp
T/S,nalidixic acid
++
V.choleras
tatracycline,T/S
++
++
giardiasis
metronidazole
++
++
amebiasis
metronidazole
++
++
++
? = controlled studies have not been done in
chiIdren
T/S = trimethoprim-sulfamethoxazole
Please note that antibiotics are indicated only in cases
of infection due to one of the four species of organism men
tioned in the table.
Surface Antibiotics - Dike colistin sulphate (e.g.
Walamycin suspension) or soframycin (e.g.Sofrakay) have no
role to play in the management of diarrhoea.
A special word
6
-6of caution about Neomycin is essantial.
(Market preparation
are kaltin - Neomycin, Renokab, Combactin) Neomycin not only
causes renal damage hut also makes diarrhoea, dehydration and
nutritional losses Tbrs".
It also interferes with ORS absorp
tion - (h)
Chloramphenical alone or in combination should not be ■
used since it is not the drug of choice in acute diarrhoea
and can kill patient by its toxicity.
It should be reserved
for typhoid fever, (market preparation are Chlorostep,Ifistrep.
Enterostep etc.)
IV) Antidiarrhoeals :
Role of Binding mixtures - Like kaolin and Pectin or
Bismuth and. Kaolin (Market preparation are Pectokab, Rocklin,
Linopec, Chloramhin suspension etc.) have no place in manage
ment of acute diarrhoea- (i)
They may solidify the stools.
But the basic pathophysiology is not altered. They tend to
hinder the absorption of antibiotics.
Nothing is gained
beyond a false sense of security.
Solid stools does not mean
that loss of fluids has been stopped.
Role of Antimotility agents - (market preparation are
Lomotil, Imodium, Imosec, Lomofen, Loperamide)
These drugs
also have no role to play in management of acute diarrhoea.
Diarrhoea protects the body by getting rid of organisms
through increased peristalsis.
These drugs by reducing the
peristalsis hamper this protective function. They merely
create a false sense of security by reducing the frequency of
passage of stools.
But the outpouring of electrolytes and
vzater into the intestinal cavity is not arrested.
Lom?.til
has recently come under severe criticism.
It is dangerous
drug because
(1)
It m-sks the fluid loss — (j)
(2)
There is a narrow range between therapeutic and toxic
dose of lomotil - (k)
(3)
There are abnormal sensitivity reactions found in
children - (1)
(4)
It increases the febrile period, of disease and prolongs
excretion of bacteria in stools.
Lomotil is a dangerous combination of drugs-contraindicated for children under age of 2 years and probably never
indicated in childhood diarrhoea.-(m)
Antispasmodics - should be used when colicky pain
accompanies diarrhoea.
•
Cliocuinols
(lodochlorohvdroxvquinolins)
The common market preparations are Mexaform and. Enterovioform etc.
There is no evidence to suggest that cliocsuinols
are effective in prophylaxis of travellers diarrhoea. - (n)
In fact it seems that if large dose^’of clioguinols can
produce severe and clinically obvious neurological-damage;
the accepted smaller dose schedules may cause suhclinical
neurological damage, -(o)
Popular commercial preparations
contain one antimolill'typagent besides clioouinol.' This
reduces the frequency of motions though clioguinols are not
useful in diarrhoea.
7
-7The companies deny that the neurological damage is common
outside Japan.
This is unconvincing because ciiocuinols indu
ced neurological damage has been observed outside Japan and
identical abnormalities have been produced in animals.
These
dangerous drugs are still available as over the counter drugs
in 109 countries.- (p)
Oas Market ?reparations - Orlyte-, Orhydrate, Ptolyte are
the only ones which come close to the WHO recommended formula.
Others like Emlyte, Lactolyte, Electrol, Electral forte do not
confirm to WHO recommendations and are expensive as well.(s)
'The present task before us includes (1) Finding out %
of cases of acute diarrhoea in India attributable to each
pathogen. (2) Popularising the use of ORS in doctors as well
as lay public.
(3) Making people and doctors aware of the
misuse of antibiotics in non-specific diarrhoea.
gEFERBNOBS.
(a)
(b)
(c)
(d)
(e)
(f)
•
(g)
(h)
(i)
(j)
(k)
(1)
(m)
(n)
(o)
(p)
(r)
(s)
WHO/ODD/SER 89.2
Tropical Gastroenterology Vol.l Jan - Mar 1939
Diarrhoeal diseases in infants and children ICMR—1979
Where there is no Doctor
International Study Group 1,931
International centre for Diarrhoeal disease research
.Bulletin.
Journal of Tropical Medicine and Hygiene 33(1) 43- 45
Population report 1930
WHO/ODD/SER 80.2
Medical Letter 1930
Clinical Paediatrics Jan 1973, 47 - 49.
American Family physician Oct 1976.
Paediatrics 1930
British National Formulary 1931
Paediatrics 1974, pg 339
Lancet 1976 Editorial
Medico-friend circle Bulletin, June 1932
Note on commercial antidiarrhoeal preparations by
Shirish Datar presented at the VIII Annual Meeting
of Medico-Friend Circle.
Glossary- For non-medicos
Pathogenic agents - disease-causing organisms.
These could he viruses or bacteria or unicellular orga
nisms like amoeba.
Virus - These am the smallest living organisms not
seen by ordinary microscope and are responsible for a great
variety of illnesses in human Moines.
As yet scientists have
not been able to invent safe, effective drugs to kill viruses.
Common cold, smell-pQX, chicken-pox, measles, infective
hepatitis (jaundice) imjn.ps, po.limyelit.is - are caused by
different types of specific viruses.
These diseases are gene
rally self-limiting and antibiotics have no role to paly in
such cases.
About half of the diarrhoeal episodes are caused
My viruses - Rota virus is the commonest variety involved.
Shigella, Salmonella, E.Coli -.etc. are names of diff
erent species of bacteria.
..3
—3Protozoa Acidosis in blood.
Organisms consisting of one cell.
excessive concentration of acidic substances
Shock - A serious life-threatening condition characteri
zed by extremely low blood-pressure and great disturbances in
the normal function of the body.
Coma- Life-threatening unconsciouness
Paralytic ilens - Paralysis of normal rhythmic movements
of the intestine (ileum- a part of intestine
In vitro -
In laboratory conditions, outside the human
body
Peristalsis. — normal propelling movement of the
intestine consisting of *******ifii>
^
*
************** A
* *^t
***
^
-.'c
****
*
*
*
*
*
Five "D"s of Diarrhoea treatment
1) Dehydration - this must be corrected as a top-most
priority
*
2) Diagnosis aetilogical - is important to decide
whether and which antibiotic to be used.
*
3) Drugs - appropriate and only when necessary
*
*
*
*
*
*
Zidecuate nutrition is vital to avoid *
downhill
course to death.
*
*
*
4) Diet -
*
*
*
5) Disaccharidase deficiency — seen only in a few
patients, but one must keep this possibility in
mind
*
*
*
****** je#-ft t'c Jr ■& it «■ * * * £ -Ait x
iti'f *****
☆ **** «;•
x
*
5fc
**
<:^
***
•A
-rhythmic contraction and relaxation.
This movement is respon
sible for the slow,forward movement of food.
disaccharidase — an enzyme which breaks down dis
accharides like sucrose (ordinary sugar) into simpler mono
saccharides like glucose.
intravenous therapy - Treatment by injecting fluids,
medicines in the veins.
binding mixtures - These mixtures are supposed to
"bind” the loose stools, thus rendering them solid.
Zkntjmotility drugs - These drugs reduce the motility
(movement) of the intestine.
Intestinal contents are there
fore retained for a longer time.
Zjntisoasmodics - These drugs relieve the spasm of the
intestine which is responsible for the colicky pain in
diarrhoea.
9
-9—
GJ -J
.J 9..'Jg
.‘.'..‘-AjJ.JOJ.?'JiJGGJGJ..
*
j©bGJJ©JGJ©jJGJ©J
©
©
©
Only 13 out of 51
Comnercial Antidiarrhoeals found useful
©
Nitin Sane a Mi?C-suhsribor in Pune has studied 51 ' ©
commonly used commercial preparations sold as antidia©
rrhoeals to find that only 13 of these contained ingre— ©
dients (in adequate doses) whose efficacy has been scien-©
tifically proved,
fhe rest 38 preparations were, found
©
to be useless on account of various reasons.
Their
©
breakdown is as follows ■
©
©
©
©
©
©
©
©
©
\j)
Preparations which "'re useless because they contain ©
©
©
a)
b)
©
c)
©
©■.
d)
e)
©
©
©
f)
©
drugs in insufficient amount—20 products
irrelevant drugs (for example chloripheniramine
malente) 9 products.
drugs of doubtful value
(for example—pectin,kaolin) ...25 products
drugs not .indicated in diarrhoea
(for example-streptomycin ...21 products
drugs which should not be used on account of.
their.' toxicity
(for exnmple-diodoquin) ... 11 products
drugs in wrong combination,
(for example combination of chloramphenical with
streptomycin) .... 14 products.
':-
©
’
-
¥
©
©
©
Many preparations contained more than one of the
undesirable features mentioned above.
,
©
©
Preparations containing metronidazole,furazolidone
were considered ns useful even if these were accompanied
by ingredients like pectin, kaolin which are of doubtful
value.
©
©
<J
G
@
©
©
@
@
©
©
©
@
©
©
©
©
©
The preparations given in the November 1931 issue
©
of Monthly Index of Medical Specialities (MIMS) under
1 .*
the heading "antidinrrhoeals" ware taken for this study. G
MIMS has not included single-ingredient brands of hmpi)
cillin, trmethoprim—sulfamethoxazole etc. in this list. .0
The above analysis is based on the~latest editions of
©
Textbooks of Pharmacology by Gaodman-Gillman,Martindale
G
etc.
’ ©
pJ
©
©J©©©
7.J j j Jp,
J.)XJj J ,’J
XAJJjQ©
Towards rational drug therapy of diarrhea
Mark C. Steinhoff, Vellore.
TABLE I
Antidiarrheal Therapy
Type
Efficacy
Example
Side Effects
Adsorbents
Kaolin,pectin,
attapulgite, bismuth
salts.
0
adsorption of
antibiotics and
other drugs
Anticholinerqics
Atropine,hyoscyamine
0
Salivary,ocular,and
cardiac parasy
mpatholytic effects
Opiates
Codeine,tincture of
opium,Lomotil
+
respiratory depre
ssion, coma,
prolongation of
shigellosis
Lactobacillus
Yogurt
0
none
Absorptionincreasing
oral glucose-electrolyte
fluids
+
Hypernatremia
possible
Secretion
decreasing—
(experimental)
Aspirin,
chlorpromazine ■
+
salicylate toxicity
hypotension,
dyskinesia.
TABLE II
ANTIMICROBIAL THERAPY
Organism
Selected Antimicrob
ials .
Campylobacter sp.
Erythr omyc in
Decreased
duration/
volume of
diarrhea
Decreased
duration of
positive
culture.
? +
? -r
Escherichia coli
enteropathogenic ampicillin,
enterotoxigenic
T/S
tetracycline,T/S
+
+
? +
? +
0
Salmonella spp
chloramphenicol,
ampicillin
neomycin ,amoxycillin
0
Shigella spp
T/S,nalidixic acid
++
V.cholerae
tetracycline,T/S
++
++
giardiasis
metronidazole
++
++
amebiasis
metronidazole
++
++
? - controlled studies have not been done in children
T/S = trimethoprim-sulfamethoxazole
2
I
• TABLE III
x
Recommended aitimicrobial. Therapy for specific
Organisms
Shigella species
Trimethoprim - sulfamethoxazole
10 mg T + 50 mg S/kg/day in two doses x 5
day (max.320 mg T + 1.6 gm S/day)
Nalidixic acid 55 mg/kg/day in four doses
x 5 days.
V. choleras
Tetracycline 50 mg/kg/day in four doses
(max 2 gm/day)
x 3 days
boxycycline 6 mg/kg once
(max 300 mg)
Trimethoprim - sulfamethoxazole
10 mg T + 50 mg S/kg/day in two
doees x 3 days
(max 320 mg T + 1.6 gm S/day)
Giardia Iambiia
Metronidazole 15 mg/kg/day in three doses
x 5 days
(max 750 mg/day)
Furasolidine 5 mg/kg/day in four doses
x 7 days
(max 1 gm/day)
Zanoebiasis
Metronidazole 40 mg/kg/day in
four doses x 10 days
(max 2.25 igm/day)
i f ii, j
hMi
■
A contribution to the discussion.;
T.B.
and society
— B.K. Sinha.
The writer wishes to unite with other participants in under
standing and taking the necessary steps leading to the elimination
ct' T.B.
Although significant strides in medical science has under-
linec. the fact that almost every body has a chance of winning the
battle of life and health against T.B., yet is continues to take a
heavv toll of men in productive age.
The situation is worse in
countries like India.
Technical and scientific mastery over the disease and people
succumbing to it,
is a reality of our social
often helplessly,
This phenomenon,
life,
a unity of opposites.
like every thing that exist in nature is
All things contain two contradictory aspects
and have contradictory, mutually exclusive,
opposite tendencies,
constantly struggling against and getting transformed into each
other,
leading to the dissolution of the phenomenon i.e.,
resolut
ion of the contradiction and transformation thereby of the
phenomenon itself.
We must therefore ask the question; why do people succumb
to T.3.,
in a situation in which it is claimed that the drugs
have been discovered to eliminate the disease ? Is the claim
unreal ? Is the treatment and drugs with which to eliminate the
disease do not reach those who cuccumb tc it ? or,
are there
inadequacies which are being ignored ?.
Let us go into these questions further.
The claim of mastery
over the disease presupposes that the causes leading to the disease
are fully understood and that all of them are accounted for in
the treatment leading to its cure.
But bodily process are
of looking at human organism.
Besides, the very instruments
we use can impose a limitation on the kind of information we
can obtain.
Moreover, with the advances in science, unknowabi-
lity has become a factor in complex computation.
In view of
all this it may not be scientific enough for a scientist to
point physical cause of the disease.
On the other hand,
there is growing awareness to define
health as a state of physical, mental and emotional well being
rather than a mere absence cf disease and infirmity.
It means
that any thing that distrubs the harmonious functioning of
physical,.mental and emotional functioning of life should be
considered as a cause cf disease.
It is therefore easy to see
that the greatest source of such imbalance lies in the relation
... 2.
BANGALORE - 560 001
neurophysiology.etc. and no unifying theory has
COMMUNITY HEALTH CELL
like biochemistry,
keen put forward in medicine that interrelates all these ways
47/1, (First Floor)3t. Merits Road
understood in a number of narrowly defined and distinct terms
:
2 :
that men enter into with ether men in the society in the process of
production end exchange cf material and spiritual values necessary
for man's existence and growth.
It is this relationship which is
at the roots cf the needy and the sick not getting the treatment
and cure : the reality of cur social life.
Various aspects of. .the reality of cur social life have been
sufficiently debated and desribed from various angles providing
quite a lot cf information.
Some of them is given below;
- Most of the people are forced by the circumstances to live in
extreme poverty.
■ Govt., policies concerning handicrafts and small scale industry
is such that most cf them must keep consuption or wages at the
lowest failing which they must go bankrupt.
- The percentage cf Govt.,
expenditure on public health and other
services of public utility has been declining.
It means that
fewer and fewer people are in a position of availing these facili-
ties.
"
It means that those able to avail these facilities must
have links with the rich and the powerful or must have sufficient
means to bribe the authorities.
The other part,
services from the private institutions and
professionals are prohibitively costly and ruinous to the people.
- Taxes by the provincial as well as the Central Govt, has been
increasing and theratic cf direct tax to the indirect tax has
been decreasing.
It means that the pocrer ones are more heavily
taxed forcing them further deep in poverty and want.
-■ Dru3 manufacturing companies are extremely exploitative and
profit oriented and go to any length for profit including the
advertisement and sale cf useless and harmful drugs at exherbi-
tant price.
And in this the Govt.,
often lets them do what they want.
If we look mere closely then we find that all these aspects
of the reality are interrelated and that they together serve the
interests cf those who control the means cf production either
through individual ownership or through the Govt.
give rise to another question ;
This ought to
is it possible that the claim
that science has advanced to such an extent that a disease called
T.3., has been conquered and therefore no one need succumb to it
is put forward to serve this very interest ?
it seems to be so.
Otherwise, the reality of the disease having been conquered and the
disease taking its toll cannot exist side by side.
This drives us to a conclusion that on the one hand we should
lock into the very claim critically,
i.e., whether or not the claim
cf having discovered the remedies for cure of T.B.,
is correct;
and on the other hand look for an alternative system of cure
which will net suffer from the same antimony i.e., which all can
get xf they so desire.
Another conclusion underlines the need of
3 :
s
waging a struggle against the social reality in which people
succumb to such disease which are thought to be curable.
This should also be kept in min' that any single work of the
above outlined can achieve its objective in isolation from the
rest; all the three must go together tc give the desired result.
Homoeopathy seems to offer'the solution.
It not provides a
frame work for looking into the claim critically,
i.e., whether
or not the remedy for T.B., has been discovered leading to its
elimination but it holds the premise of making the remedy universa
And what's more,
lly available in terms of cost.
it can be a tool
in the hands of the people struggling against the exploiters by free
ing the people and the Gentry's economy from the chains of exploitat
As manufacture of these drugs does not
ion to a great extent.
require such technology in which monopoly can be established lead
ing tc super profit and monopolistic exploitation.
But it is not only on these ground that this system of
Important though these grounds
treatment is being recommended.
are, but in the context of treatment,
its recommendation rests
primarily on its effectiveness in practice.
Many case can be
cited tc demonstrate the effectiveness of this treatmen but I
cite only twe examples :
1.
Mrs. S.K.Suman Khcnkar,
40 year cld,
from Sonegaon Wardha,
came to Dr. Bhongade with following complaints:
Buring in throat,
ion,
dry cough,
amelioration with cold applicat
rigour of chill, recurrent coryza, pain in
feverish,
chest, both apex, more in left with pain in back,
at the region of scapula,
agg.
slight cold,
sour food,
salt water gargling,
tea,
left side
stitching and ulcerative pain,
slight air current,
amel.
lying down, hot application,
pain in chest aggravating while coughi.ng, weakness with
trembling of whole body agg.
at morning,
Rhumatic trouble, pain in lumber regitn,
both sides, more in the left side,
changing sides,
pain in legs,
at circumscribed spot,
aggr. during sleep; touch,
amel. hot application;
spasm and rhumatic
agg. sitting, during sleep;
ion. Burning pain in soles,
amel. after eating;
amel. hot applicat
eyes, cracks in soles;
Thirst : more than normal.
Sleep :
d.reams :
disturbed easily,
sleepless for 2 hrs.
fearful, weeping during sleep.
after 2-3 am.
History of profuse
menses, presently having early menses after 3 weeks lasting
for 4 days,
clots,
intermingled with red blood.
Lukeria : milky and thick,
after loop insertion.
Had,suffered from T.B two times, both lungs were affected.
Unde too had T.B.
Children very succiptible to cold and coryza,
lasting for long.
4
Physian's observation ; lean and thin
Temp. 98.5; Pulse 88 ; B.P.
110 - 60.
Weight : 41 Kg. on 11.9.'81.
She came to the Doctor on 9.10.'80
Treatment : Phosphorus 200
1 close . •
Blood and Sputam tests on 14.10.'-80 indicated s
Blood ;
H.B.
59
P.
51
L .
45
E.
3
T.L.C.
9600
Sputam :: A.F.B. Positive.
Treatment :
18.10. 1 80.
Phosphorus 200
.. -
1 dose
n
11
1 . 11
26.11.
Complained loose motion
Aloes 200
3
doses.
30.11.
Aggravation of cough, sleepless after
3 - 4 am., no remarkable change
Phosphorus
Im
1 dose
13.12
More cough, harshness, pain in chest,
tickling in throat.
Rumex 200
1 dose.-
■
16.12
17.12
n
11
Constipation,
ineffectual urging
Nux Vomica 200
6 doses
30.12
Cough aggravated, tickling in throat,
pain in abdomen and chest
Phosphorus
Im.
1 dose
15.1.1 81.
Cough reduced. Pain in left leg,
constipation, ulcerative in vagina,
sensitive to cloth
Phosphorus
Im
1 dose
13.3.
Teeth ache,
Phosphorus
1 .7.
Feels better
Phosphorus
lm
18.7.
Cough agg. tickling in throat
Kali carb. 200.
11.8.
Kali carb.
20.8.
Feels better, back ache, cough day time
Sujbphur 200
19.9.
Alrcund improvement
Sulphur im
1 dose.
Improvement continues.
cough
lm 1 dose
1 dose.
200.
The patient is cured.
Blood and sputam test were done in the meantime,
Blood :
H.B.
P.
L.
E.
T.L.C.
Even after the cure,
some more -Hm"
56
56
41
3
11200
Sputam ;
on 16.4.'81
A.F.B. Negative
the patient was given cjlobles for
:
2.
5 :
The other example is from Mozart,. a village in Amravati
District. Ratnamala Tat Shelke,
38 year old,
came to Dr-.Gumble with
following complaints ;
extreme weakness, cough, white sticky but easy expectoration,
aggravation of cough after delivery and in the summer,
burning during urination,
satisfactory motion,
sweat on chest,
thirst,
urine yell', w, worms in steel, un
loss of appetite,
face and head,
continious feverishness,
regular 5 day menses.
Had been treated for T.B. before.
Physician's observation ;
Treatment ;
lean and thin, slow.
26.5.'84. Nitrum sulph
3 doses
200
4 times daily for 2 days
1 .6.
Rhustox 30
3 .6.
feels better
Tuberculinum 200
29.6.
Improvement continues
Tuberculinum
200
14.7.
3 doses
Complains cf cold, loose motion
Nitrum sulph
lm.
3 doses
30.8.
Complains subsided
Tuberculinum
lm.
30.11.
feels much better, put on weight,
feverishness, weakness reduced.
Nitrum sujbph 1000
3 doses.
3 doses
cough
Blood test shows the reduction of Esnophelia
and increase of Hemoglobin
The patient is almost cured but the treatment is
continuing.
There are many more case histories of treatment that can be
cited for the proof of effectiveness of this treatment.
But the
difficulty is that pathological tests have not been dene and there
fore the kind of proof that is demanded from them is net available.
And the reason for this lack cf pathological test is that homoepaths following the logic of homoeopathy do not believe in
pathclogical tests.
Moreover, these twe case histories will reveal the difference
of medicines given to patients.
The reason is that hcmceopathy does not believe in entities
called disease (S).
It treats patients'
totality of symptoms
rather than a small group cf them which give rise .to such entities.
After all,
they merely represent an arbitrary selection cf certain
manifestations cf illness that appear together with a certain degree
cf frequency.
Prescribing in homoeopathy iS solely based on actual
observation cf the effects cf homoeopathic medicines on healthy pers
ons.
Such observations define the range cf the action cf the medi
cines and provide all the information needed to help select a proper
remedy fcr an individual patient.
But this does not mean that the
suitable medicine for the symptoms categorised as T.B. could be any
one from some 2000 odd proved medicines listed in Materia Medica.
6
;
6 .
Paradoxical though it may sound, but there are nearly 20 medicines
fr?m which the most suitable medicines for most patients can be
selected depending on peculiarity of symptoms in individual cases.
Then there is a ncsode, Tuberculinum, which is fcund to be
helpful in many coses when there is a history of infection either
in the individual patient or in his parents.
Although routine
prescribing of the nosodes along isopathic lines is not consider
ed to be good homoeopathic paactice,
it can be of great help.
It
can be used not only to break up the lingering effects of the
disease, but also to reach deep into the constitutional pattern of
a patient and clear a chronic miasm that may have been implanted
long before through exposure.
It builds body resistance against
the disease.
Homoeopathic treatment holds a great promise for the suffer
ers and those interested in removing the causes of suffering but
gfe
all this lies, buried in the heaps of abuse and ridicule against
£
homoeopathy.
It is true that advocated of homoeopathy and homoeo
paths themselves provided some basis for it,
and did almost nothing
to counter and expose the abuse and ridicule, most of which is
motivated not by science but by counter-science.
But neither evidence
nor logic has been put forward to refute the basic premises of
homoeopathy.
On the contrary,
fresh insights have been gathered
from laboratory experiments to uphold the effectiveness of the
system and cure.
Science is defined as "the cognition of necessity".
prime task,
therefore,
Its
is to investigate and analyse the needs of
the society and to pave the way for its fulfillment.
The signifi-
cance of a scientific discovery depends solely upon its importance
to society in the context of its needs,
towards its needs.
gfe
a
and the society1s awareness
But the social needs and its awareness often
depends on the recognition of the class in power.
It is they who
decide what constitutes social needs and use the resources under
their command to fulfill it.
If their policies produce such results
which are contrary to the social needs then it reflects a stage of
development of the society in which the ruling class in existence,
its ideas and theories,
its forward march.
live.
its '
science 1
cannot lead the society in
This precisely is the situation in which we
In such a situation,
the essence' of science consists in
taking the theory forward by basing itself firmly on such experience,
Such data obtained in pracice which articulate and meet the social
need in a better way but are not considered 1scintific1
ruling 'science'
take up.
of the day.
enough by the
It is a task that society will have to
My submission is that homoeopathy should be examined in this
context, I hope, M.P.C. will come forward in doing the needful.
OoO
B.K. Sinha,
C/o Dr. M.N. Gumble,
Gurukunj Ashram,
Dist. Amravati, Pin : 444902.
X>'q. $•, p.Tgko^
P -k-—
New Drug Policy
Betrayal of Consumers’ Interest
The following is a statement issued by the Voluntary Health Association of India on the new drug policy. It
was issued on September 16, 1994.
_ Editor
he Voluntary Health Association of India
(VHAI), New Delhi, a federation of more than
3000 organisations involved in community health,
has noted with great concern the announcement of
the new drug policy. The policy was announced at a
press conference on September 15, 1994. We are
shocked that the policy is totally in the interest of
the industry and the consumers basic needs are
neglected. We are dismayed at the 'callous
indifference of the government towards the health
needs of the people. Even the way the government
chose to announce the drug policy (which has farreaching implications as far as the health and life of
millions of people are concerned) through a press
conference rather than after a proper discussion .in
Parliament itself is undemocratic.
Drug prices will shoot up because the number of
drugs in the price control range has been brought
down to 73 from 142. Increasing the profitability
ceiling for bulk drugs will directly further worsen the
situation as far as the prices are concerned.
The rationale for allowing price decontrol, can in
no way be justified if the figures for the last few
years are studied. There has been
a steady
increase in sales, profits and dividends of the drug
companies. It is sad that the government has
‘bought’ the drug industries’ argument that drug
production is not profitable and drafted the policy
accordingly.
The total liberalisation will further worsen the
existing anarchic situation in drug production. In the
absence of a mechanism to ensure the production
of essential drugs, its acute shortage will hit
all national health programmes. Its implications
are far-reaching as it will lead to further proliferation
of hazardous and irrational drugs. The argument
of the industry that trade is a fundamental right
should not be at the cost of the public’s health and
H
life.The present policy as such will open the gates
for the multinationals. High priced, useless and
2.
. their . organised; sales promotion propaganda with
advertising and marketing strategies will leave no
chance for the medical profession to make a free
decision. Furthermore, drug is not a substance
which an ordinary consumer/patient can decide
upon.
The government is giving a ‘false hope' to the
public that if required the government will bring back
decontrolled drugs to the price-control category. But
lessons from past experiences show that giving
even a chance for overcharging for drugs by drug
companies has never benefited the consumer.
hazardous drugs will be pushed down the throat of
the gullible Indian consumer. Increased dependence
or imports, higher prices and proliferation will
happen due to the policy which allows companies
with 51 per cent equity participation to be treated on
par with Indian companies. This policy will further
hit the Indian industry resulting in import and
increasing prices.
It is further disappointing that there has been no
reference to the irrational and hazardous drugs
which are still being sold.
The neglect towards such an important issue
where thousands of products keep the life of the
public at stake is very critical. These products will
pose a continued threat as far as safety is
concerned because the new drug policy does not
address this critical issue.
Even in developed countries where the industry
has been enjoying a “free-hand” it is under criticism
of late. The spiralling costs in health care in the
USA is just an example. Recent studies from
different parts of the world point out that competition
by market forces need not bring down prices. For
example, the Office of Technology Assessment in
the US was forced to study the costs and profits of
pharmaceutical manufacturers because of the ever
spiralling drug costs.
The OTA report dated Februray 25, 1993 tells
why drug prices in the US are high and it also
shows that competition simply does not work in the
market for prescription drugs which are becoming
unaffordable even in the US. The OTA report states
that to reduce prices there is room to reduce profits,
advertising costs, unimportant research while leaving
breakthrough drugs intact and leaving industry a
generous profit.
These lessons of failures of such liberalisations
and Structural Adjustment Programmes initiated in
those countries, instead of being taken as an eyeopener, are being ignored.
The drug companies have proved in the past that
September 24.1934 ■ MAINSTREAM
The VHAI urges the government to reconsider
the policy. We urge that a “rational drug policy"
which will ensure the concerns of the consumers
[namely, (a) availability of essential and life-saving
drugs, (b) withdrawal of hazardous and irrational
drugs, (c) adequate quality control and drug control,
(d) technological self-reliance] is to be formulated in
a democratic process by discussions at various
levels, like with professional bodies, health groups,
rational drug groups, consumer groups, voluntary
organisations, people's organisations and in the
Houses of Parliament.
■
BUSINyss
DRUG POLICY
Writing
A New
Prescription
...to stimulate fresh
investment but the
industry is not impressed
By SIIEFALI REKHI
HE new drug policy is only the
beginning ofa longcourse of medi
cine aimed at removing the mal
aise in the industry. While the Govern
ment feels it will radically free
pharmaceutical units from hurdles in
the form of price and entry controls, the
industry has received it as a mixed bag.
Says Parvinder Singh, chairman
and managing director of the country's
second largest drug firm. Ranbaxy Lab
oratories: "Though the policy is far from
perfect, now we know in which direc
tion we arc heading." The new drug
policy is likely to stimulate fresh invest
ment. an influx of the latest technology.
the introduction of new drugs and
higher rates of return.
The last policy failed to achieve any
ofthis. Announced in 1986 and followed
up by the Drug Price Control Order
(dpco) in 1987 imposing restrictions on
drug prices, it was bitter medicine for the
industry. The restrictive pricing regime
and lack of respect for intellectual prop
erty laws dissuaded foreign firms like
Upjohn and Sterling from investing in
India while others such as Roche and
Searle closed shop.
International research showed that
of the 450 molecules—jargon for the
T
composition of a drug—manufactured
globally, only a 100 were introduced in
India. The Government’s defence was
that most of these molecules were not
required, but it didn’t have a clue as to
how many. Says Homi Khusrokhan,
joint managing director, Glaxo: "There
was no incentive to do the kind of
research required to discover basic
molecules."
But things arc changing. To encour
age investment and technology, the
new policy makes lucrative offers. In
dustrial licensing is abolished except for
the live drugs reserved for public sector
enterprises. Drug companies are also
allowed to make more money and im
prove their bottomline. Units making
drugs from the basic stage will be enti
tled to a 4 per cent higher rate of
return—at present, they are entitled to a
return of 14 per cent on net worth or 22
per cent on capital employed.
The ‘maximum allowable post
manufacturing expense’—expenditure
incurred on marketing and distribu
tion—for units making Category I drugs
has been increased from 75 to 100 per
cent. (Category I drugs are those which
are covered under the national health
programme.) A more important feature
of the new policy is that it allows foreign
multinationals up to a 51 per cent
Steps Taken
Still Pending
H Lie using has been abolished for
all drugs except the five reserved for
public sector units.
n *’ dtinational firms will be
tn d on a par with Indian compa
ny m all respects.
a The number of drugs under price
controls reduced from 142 to 73 and
the new dpco to be announced.
ra The formation of the National
Drug Authority will take time. This
may hamper quality control.
s Old data used for imposing con
trols on monopolies. Position is un
clear for new drugs.
□ Although a cess has been pro
posed, incentives for research and
development remain unspelt.
144 INDIA TODAY ♦ OCTOBER 15.1994
Mathur: "You can’t let go of all controls’
holding in their ventures in the country.
And they will now be treated on a par
with Indian companies.
It was in 1978 that the restraints on
mncs were first introduced. And they
continued without any revision there
after. According to the old policy, mncs
with a 40 per cent or above foreign
equity stake could only manufacture a
select band of 66 drugs out of a total
numberofover 500. The mncs could not
make formulations by importing bulk
drugs and had to sell a part of the bulk
drugs made within (he country to non
associated formulator companies.
These restrictions were not applicable to
Indian companies. Now. they will not
hold for foreign companies either.
But what really matters to the indus-
"The policy is far from
perfect but the industry
knows the direction in
which it is moving.”
Parvinder Singh, Chairman,
Ranbaxy Laboratories
NAMAS BHOJANI
THE INDUSTRY
Better Health
1FE has been good for the drug
industry in the last few years.
J Investment was stagnating till
1989-90—an after-effect of the 1986
drug policy. But with the new
Congress(I) regime deciding in 1991 to
allow reasonable price hikes, profit
ability began to improve. A study of 53
companies, done by the Centre for
Monitoring the Indian Economy,
shows that net profits have risen by 60
per cent for the past two years.
The production of drugs—both
bulk drugs and formulations—was
Rs 2,100 crore in 1989-90 and is
estimated to cross Rs 7,200 crore in
the current year. Experts reason that
given its current growth rate, indus
try turnover could well cross Rs MAKING DRUGS PAY
16.000 crore by the year
2000. Says Vinod
Vaish. joint secre
tary. Department
of Chemicals and
Petrochemicals:
"There is no way
any company. In
dian or multina
tional. will want to
leave Indian soil. The
Profit before depreciation
Source: CMIE
future prospects are ’ and
income tax
BK SHARMA
far too enticing."
Glaxo and Hoechst, which to bad-based Ambalal Sarabhai is negoti
gether account for Rs 900 crore in ating with American companies to
sales, hiked their stakes in Indian produce new anti-cancer drugs. The
ventures to 51 per cent, even before group is planning to invest Rs 150 crore
the policy was declared. And this to increase its Vitamin C production
capacity from 500 tonnes to 2.000
knowing the constraints.
It wasn't so in the past. The parent tonnes. With Sarabhai being one ofthe
companies of mncs here were unwill two producers, this may well cure the
ing to bring in investments. Take drug’s scarcity. —daxsesh partch
I
“The Government has
not given any incentive
in the past to do basic
research in drugs.”
Homi Khusrokhan, joint
managing director, Glaxo Ltd.
try is pricing. Price controls have still not
been removed. An independent body of
experts—yet to be constituted—will form
the National Pharmaceutical Pricing Au
thority to decide price revisions within a
time frame of two to four months.
“Drugs are an emotive issue. You
can’t let go of controls altogether." says
K. K. Mathur, secretary in the Depart
ment of Chemicals and Petrochemicals.
But at the same time he adds: "We know
that the past emphasis on price ceilings
sacrificed the quality and availability of
drugs." The new policy has retained
price controls on 73 drugs as against the
earlier 142. Price controls will now
fcffectonly47percentofthedrugssoldas
Compared to 70 per cent earlier.
OWEVER, market monopoly will
be regulated through a turnover
criterion. All drugs with a turn
over of Rs 4 crore and above will come
under price control. For bulk drugs with
a turnover of above Rs 1 crore, the
monopoly tag will apply unless there are
five competitors in the field. This, say
manufacturers, is unclear. For one. the
Government is relying on outdated data
from the year 1989-90. Newer drugs
such as the antibiotic ciprofloxacin hit
the market after March 1990. And no
one is sure how the Government will
arrive at correct turnover estimates for a
substantial number of small scale units
accounting for 30 per cent of total drug
production. Says D.G. Shah, director
(business development), Pfizer Limited:
"The data is suspect, there is still uncer-
H
Pfizer for instance. The company did
not increase its share capital of Rs
11.72 crore from 1984 but increased
production capacities which raised
sales by 275 per cent to Rs 21336
crore. This was funded entirely from
internal accruals and borrowings.
The reluctance of promoters was
responsible for the low level of invest
ment—there was no investment in
the past five years. Most of it came
from Indian companies. Companies
like Ranbaxy, Lupin. Cipla and Cadila
moved rapidly to establish their ex
port bases. And Indian exports grew
from Rs 100 crore in 1980-81 to Rs
2.000 crore now. Exports are also
likely to get a boost with companies
now well set to exploit the global
demand for generic drugs.
Simultaneously, new drugs will
move into the Indian market Ahmeda-
tainty. How do you concentrateon your
business plans?”
The other bitter pill is the proposed
cess oflpercentonthe industry which is
expected to generate Rs 24 crore every
year. It will be used to fund research and
the activities of the National Drug Au
thority, a body that has tobesetupinthe
near future as per the new policy.
It is feared that all these fresh demands
might eat into the profits of an industry
that has only started recovering from the
1986 policy in the past tw'o years. In the
'80s. the average rate of return for the
drug industry was a third of the average
for all industries put together. Says an
embittered Habil Khorakiwala. chairman
and managing director. Wockhardt Lim
ited: "The new moves will just add new
layers to the existing bureaucracy. The
Government has spent crores of rupees in
the past four decades but has made no
significant discovery."
Other vital issues still have to be
settled by the ministry. The most impor
tant being cases against several drug
companies of making excess profits in
the past. The Rs 250-crore penalties
demanded by the Government on this
front could seal their fate. If the units are
forced to pay up this sum. the question of
whether the new policy is pro-business
or not may well become irrelevant.
—with ROBIN ABREU
OCTOBER IS. IW ♦ 1X01A TODAY 145
ALPORT OF T.HE PUBLIC BOARD OF INQUIRY ON DEPO-PROVERA
17 OCTOBER, 1984
DATA AVAILABLE CN TEE LONG-TERM RISKS OF DMPA
ARE INSUFFICIENT AND INADEQUATE TO PROVIDE A
BASIS FOR k DECISION VJHETEER THE BENEFITS OF
TEE DRUG AS k CONTRACEPTIVE OUTWEIGH ITS DIS•ADVATTTAGES UNDER CONDITIONS CF GENERAL MARKET
ING IN THE USA
There ar© adequate data to assess the efficacy and
fits of DMPA as a contraceptive.
There is also
icic-nt information on its short term side effects and
s. The drug is clearly a highly effective contraceptive
certain specific advantages, and it doos not appear to
any immediate irreversible serious side effects,
ver, the facts relating to the long terra consequences of
use cf the drug are inadequate and insufficient to
ide a basis for risk assessment. This is a serious
•i<ncy in light of the specific questions that have been
od that the drug may have major adverse effects following
long terra use or that may become evident only after a
nt period. Most important among those lias been the
om over the drug's carcinogenic potential.
The long term consequences of he use of DMPA on
' a; .’.as, in particular of the breast and uterus, as well
a jc.oporosis and atherosclerosis are of particular roloe for any risk/benefit assessment of the drug's use in
United States because of the susceptibility cf the
Lotion in this country to these diseases.
~Tn the absence of adequate data on the long term
equences of the drug it is not possible to arrive at any
ntifically defensible conclusion whether or not the
fits of the drug, when used as a contraceptive, outweigh
risks..for the average healthy individual in the United
os, xt also makes it impossible to compare the risk/
fit ratio . of DMPA with that of other drugs available
contraception.
DATA FROM TEE STUDIES CF RHESUS MONKEYS AND BEAGLE
DOGS CAN NOT BE DISMISSED AS IRRELEVANT TO THE HUMAN
WITHOUT CONCLUSIVE EVIDENCE TO THE CONTRARY.
SUCH
EVIDENCE IS NOT AVAILABLE AT THIS TIME. THEREFORE, TEE
FACT THAT MALIGNANT NEOPLASI. S DEVELOPED IN TWO
SPECIES IN TARGET ORGANS CF SEX STEROIDS MUST BE
CONSIDERED AS AH INDICATION OF A POTENTIAL OF
PROGESTOGENS, INCLUDING DMPA, TC PROMOTE THE DEVELOP
MENT CF MALIGNANCIES IN TARGET ORGANS
The findings from animal tests implicate DMPA as a
intial promoter of neoplasias because;
Chronic administration of DMPA was associated with the
ilopment of malignant neoplasias in two mammalian species.
Data are also inadequate to establish effect of MPA on
bone and on the profile of plasma lipoproteins, inform
ation needed to evaluate whether the long term use of
the drug will or will not predispose th© individual to
eotooprrosis or to atherosclerosis. Cur conclusions of
Law do not rely on this finding.
~
2
-
2)
The neoplasias developed in target organs of sex
*
steroids
x
3)
There is good evidence to support the conclusion that
in both species the malignancies were drug related.
4)
There is no evidence to support the conclusion that
the effect of the drug is to be attributed only to the
administration of excessively high doses and that the effect
of lower doses would differ qualitatively from those of
higher doses.
Therefore, DMPA in these experiments exhibited the
characteristics of a potential carcinogen p-.ccq.rding to
generally accepted criteria, ; Under the circumstances, ‘to <
dismiss the findings as irrelevant to the human would require
conclusive experimental evidence of fundamental differences
among the species in the basic mechanisms of action of tho
hormone or in the responses of target cells. There is as
yet no such evidence at hand.
Specifically, there are no
data on the histogenesis of tho neoplasias nor on the
raeehanism of action of progestogens on the presumed colls of
origin of tho neoplasias in the test animals. Therefore,
there is no evidence to support the claim that tlie
malignancies developed either in c ell types unique to the
species or as a result of a species specific response of
target cells to progestogens. Conversely, data on women who
have been ojeposed for prolonged periods to the relatively
unopposed action of progestogens are inadequate ±0 warrant
the conclusion that their response to this hormonal state in
terms of neoplasias would differ in some fundamental way
from the two species of test animals.
III.
THS DATA CM THE HUMAN ARE INSUFFICIENT AND INADEQUATE
TO EITHER CONFIRM OR REFUTE THE IMPLICATION OF THE
ANIMAL DATA THAT DMPA MAZ INCREASE THE RISK GF CANCER
IN ROMEN USING DMPA AS A CONTRACEPTIVE,
The available data on the human can not provide a basis
for conclusing whether DMPA, used as a contraceptive, doos
or does not influence the incidence of carcinomas in general
or of tho accessory organs of reproduction in particular,
because;
d) They fail to provide information on an adequate number
of long term users of DMPA, or on ex-users who have been
followed for a long enough period of time. There are only
minimal data on subjects that have
used DMPA for 5
years or longer wj th most of the data reported having bean
obtained from women who have used the drug for 2 years or less,
2)
In the majority of the studios there were no controls
followed in parallel with those using DMPA. In many
studios from developing countries there is not even informat
ion on the background incidence of the diseases being
studied in DMPA users that could serve as a basis for
comparison,
3)
In a number of the retrospective studies there is
reason to question the adequacy of the record keeping on
subjects receiving DMPA and, therefore, of the possibility
I
3
of retrieving the data subsequently for any valid analysis.
To obtain the direct evidence needed to resolve the
issue would have required purposeful, systematic collection
and recording of data on users of DMPA. and appropriate
controls with consideration of the natural history of the
diseases being monitored. Not until recently have suhh
studies been initiated. Until they are completed and full
reports of 'shorn available their value as evidence is limited.
IV.
IN CASE CF CONTRACEPTIVE FAILURE WITH DMPA, THE RISK
CF Z. MOTHER GIVING BIRTH TO A MALFORMED CHILD IS
UNLIKELY TC BE MEASURABLY GREATER THAN THAT POSED BY
THE ORAL CONTPACEITIVES! The chaHCE IN EACH CASE CAN
BE ESTIMATED TC BE SMZJLL ENOUGH NOT TC POSE ZJ-T OBSTACLE
TO THE USE OF THE DRUG AS k CONTRACEPTIVE THEN OTHERWISE
INDICATED.
Data have not been systematically collected on offspring
that have been inadvertently exposed to DMPZi in utero.
Conclusions, therefore, can only be based on the body of
epidemiological data obtained on the effects of a variety of
sox steroids, including progestagens, on the developing human
fetus'. In those cases, the drugs had boon administered for a
variety of indications and at various times during pregnancy.
This is clearly a less than ideal data base, Nonetheless it
can provide some general estimate of the magnitude of the risk
Ziccording to these data the risk of various malformat
ions attributablo to protestogens for the various malformat
ions implicated is low. The rate of contraceptive failure
with DMPA when used appropriately is also low. Consequently,
the chance of a mother bearing a malformed child following
contraceptive failure can be estimated to be small. However,
because DMPA. is a long acting depot preparation, the
exposure of any susceptible fetus to the drug is likely to
bo more prolonged than with oral contraceptives.
Consequent
ly, the range of critical and vulnerable events that m£y come
under the drug's influence may also be expected to be greater
than with oral contraceptives,
.t should bp possible to
counter balance this disadvantage of DMPA. by ensuring that'
contraceptive failure is kept at a minimum "and taking the
necessary steps to avoid inuecting women already pregnant.
s with oral contraceptives this risk should not, in itself,
A.
constitute a reasonffor not using the drug if otherwise
indicated,
There-have been no direct determinations of the concent
rations of MPA in the blood of breast fed infants of
mothers receiving DMPZi as a contraceptive nor if the amount
of the drug transferred passed onto the infant is sufficient
to have a biological effect. This information is needed
before advocating the use of DMPA. as a contraceptive to
lactating mothers in the postnatal period and before it is
possible to conclude that the drug does not pose any risk
of functional teratogenicity.
CONCLUSION O_F LAW
Accordingly, Upjohn's supplemontal new drug application
for Depo-Provora (DI4j?a) sterile aqueous suspension for
intramuscular injection as a contraceptive agent in humans
does not contain reports of investigation adequate to show
that the drug is safe for use under the conditions
prescribed, fecommended or suggested in the labelling as
required by S 505 (l),
(2) and (4) of the Federal Food,
Drug and Cosmetic Act, and the information contained in the
supplemental new drug application, combined with other
information a.bout the drug, does not provide sufficient
basis from which FDA can determine that DMPA is safe for
general marketing in he United States.
~;'JT INJECTABLE CONTIV-CEFTIVES -
INDIAN -./CMEN
ESERVE A BETTER DEAL
A campaign group has been formed in Bombay to protest:
against the Drug-controller of India approving NET-BIT as a
contraceptive. Two companies - UNICHEM and GERMAN
REMEDIES - have been given licences to manufacture this
drug.
Today's pretest demonstration in front of Oberoi Towers,
where- the Family Planning Association is holding a closed
door conference of 'exports' on NET-EN. We plan to continue
with the campaign and expand it to include all -longact; ng contraceptives,
‘•Jrat are Injectable Contraceptives? Injectable Contracept
ives ( I c) prevent pregnancy more or less in the same way
as oral contraceptives. But they are administered by
ix.Joction and are 1 ong-acting. The best—known ones are
Depo-provera and NET-EN
Depo needs only one injection
every 3 months and nET-EN, one every 2 months.
Population control, enthusiasts consider injeotables
the ideal, form of contraception for women in the thirdworld because of the ease with which thex can be administered
on a mass -scale and the low failure rate. To those who
lock at wornon in the third world as nothing but faceloss
i-.oto-:’. io bo considered in any strategx of population
control thox cook up, the benefits seem overwhelming and
the 'risks’ in terms of women's health negligible. There
has been a concerted campaign lately to 'sell' the idea
of I Cs through the media and elsewhere. The conference
organised by the Family Planning Asrociation on 28th and 29th
Decomoer 1984 at Cboroi, is part of this 'marketing
strategy'.
D o oo_- prey ora and NET-EN -the controversial contra cop t iyo_s
Depo-provera has been the centre of a fight between
Health groups and women's groups o'n the one side and
Pharmaceutical, companies on the other since the sixties,
when the Upjohn Co, of USA sought approval for it in the
sixties, Upjohn has fought a hard and long battle in the
U S unsstod ess fully. They desperately wanted approval
before their exclusive rights on the drug expired. The
campaign in the U S and elsewhere brought Depo-provera a
'bad name'. Approval for its manufacture has not been given
by the Drug-controller of India. But neither has__any
cjqolnnation boon, iven to the public or to interested
Kt.?.11®.-- ■a.7-3OU~{:. why it has not been approved ,
Meanwhi 1 o,
NET-EH another I C about which not much is known has been
approved in India and licence to manufacture it has been
granted to two companies - Unichon and Geripan Remedies.
Both Depo-provera and NET-EN have been used in India
for several years now for research purposes. This research
has been carried out maily on poor women by volunta.ry
agencies who conduct community health programmes, under the
supervision of the Indian Council of Medical Research. The
reports of the studios have not been published till today
2
and ICMR has refused to make it available to anyone. All
interested parties are supposed to take their word for it
that while Depo is not so good, NET-EN is just fine. Past
experience with contraceptives and other drugs does not
inspire in us any such trust or confidence. We believe
that we have a right to know the details of the research
studies, to make our own investigations and to come to our
own conclusicns, We do not consider the masses of women
mere pawns in population control strategies to whom cont
raceptives are 'sold' on the basis of incentives, without
prior information.
What we do know about ICs is quite disturbing. Upjohn
Co., conducted two animal safety studies in the sixties a seven year one on beagle dogs and a ten year one on
monkeys. Within three and a half years of the dog study,
all dogs on high doses and half on low doses were dead due
to inflamation of uterine lining.
(The two on low doses
who survived had their uteruses removed.) All control
dogs ane survived except one which died of bite wounds
and four which were sacrificed by the researchers. The
dogs also developed cancer of the breasts, drug-induced
diabetes and various other problems. At this point, Upjohn
declared that beagle dogs were not the ideal animals to
judge risks to human females. Later even the monkey
studies in which cancer of '(he uterus occured were said to
be 'irrelevant to human experience'. The history of this
controversy has been marked with disinformation and a
desparate desire on the part of the company to maximise
profits without making sure first that the drug is safe.
Breast cancer, two types of uterine cancer, serious
menstrual disturbances^and masculinisation of female foetuses
are some of the serious effects of Dopo-provera, Others are
depression, decreased libido, nausea, dissiness,(weight
gain without any increase in nutrition)etc,
The W H 0 report on I Cs (1982) says that the
majority of women on I _Cs_ have their menstrual cycle disrunted. The extent of disruption is stunning.
"loss than
one third of women on Depo report having any normal
menstrual cycle during the first year of usage' and
■approximately half the users ( of N3T-EN) reported at:
least one normal menstrual (cyclc during the first year',
Beth the above quotes from the W H 0 report are examples of
the concerted attempt to underplay the dangers of I Cs, In
fact, a significant number of women stop having their
periods only to haye severe bleeding after injections are
withdrawn while others bleed every day of the month while
on the drug. But everyone concerned seems to feel that
it is a minor side-effect. For Indian women who hold the
world record for anemia, it is a very very significant
side effect.
There is far loss information available about NET-EN
on human metabolism, on infants exposed to them through
breast-milk or about their carcinogenic properties. No
one seems to know why the majority of women on these
drugs suffer from menstrual chaos, -No do they know why
these women put on weight without more nutrition or why
they are depressed.
3
Yet the advocates of I Cs , including the W H 0, consider
them an ideal form of contraception. Their favourite
phrase is risk-benefit ratio. According to them if the
benefit outweighs risk, the drug should be used.
BUt_ tire risks are taker, only by women. The benefits are
main.yr;for the pharmaceutical companies, the population
control experts and the Government_s_ of third world .count ries.
There is a lot that is wrong, with our family pl nnm'ng
'policies. Its always our families and their plans. A
beginning must' be made somewhere to correct theip. Lets
start with the newest strategy which is about to be imposed.
on the masses of Indian women, Lets struggle against the
■J-iwndnti on of this country with ICS.
CUI; DEI-’L'.IJDS;
;) Ban all long-acting contraceptives and
withdraw approval for NET-EM.
2)
Make public all studies in India an Depo and NET-EM.
immediately.
3)
Stop experimenting on third world women with hazardous
drugs and contraceptives.
1)
Institute a public enquiry on the controversial
injectable and implanted contraceptives.
•jc.'lf US III OUR STRUGGLE FOR A BETTER DEAL FOR OUR WOMEN.
Campaign group against long-acting contrqcbptives,
1.
Forum Against Oppression Of Women.
2)
Women’s Centre, Bombay.
3)
Medico-friends' Circle.
4)
Stree Mulct! Sanghatna
5)
Sangharsh Vahini,
uJoUf^ rfda uo3i©rtsJd tft3o xte6
WcSdci^ wd-raerijioddraji
<^Sy^3 I3sy^!
jiDcit&ic^ xioj^rt RKitprtJS,
^3jgja6 ^±sd d^jjrfe
a^adOsx,
GoCaos cud 22 ddrd eross/o
cadd. fnrtdjddQ oic6 id?
nesiJcd^, iox Mt sodxtddQfJ. 3f£
iCoi; doujjs^co dxoddi^cj ats arfrt
crfjijO—id^jrjrd,
L3 8 acd-1
dbXdO
EX>odjs;aS>.
trodlS
adddiS},
dsouoaoa
a/.s. d.d.
a^3/
<xaaesod3x
1985 tsXoucr'S^ add ddd qd.
25eSa sidedo dtaodart 3Ccd s£eu?Ac£
dt^ruA Xrj, ud&da
*
dKdcxcdab.
*ts. 29 zd; 1988
rt5raa»S)®rt>43dart KSsSo 19860 abt^
csjss, aOj-jta ao33i. asao
24 Ood> 03 Sadcioid'toc^
75 Sard ds^ic4' 8r{/s uadiS^rofi
d.d. u^Sp&O, ds^wddfd uobdiirioiu
■^cj. KridO 28 Ood> ^d_dorjp3ci>? X3f^.
^dt 0e3 ddies aoq!. ^ekod djiei.
od, ^WJod. eajc? bjideo1' Stiy a?rt
aodt}, adds), 14 dioa at^c»rt>3idt
a^Cb d.d. ux,3cl>^, sscadTidhdcb.
d?d ded dodySdcb- d«d dtd asdradg/i
0^3/ uoddda- 3at!rt aode OsicdiJ,
dsdra ciooudcfc. aado 248 «^3,
addja64
^ado 6 d.ovrt 14
dooacdd^, idc/i3i.
rtra aljbod>3jddcl> di o?3 'ui^sdoa
&f5jw
woa
djsda
d4«do3'
xscS)3j5dhan ox3odi6^ roassddi^sj.
□jjcc/id ad3rt 3s?d»>ri
ad
sdcJj^/jdi djscf. tia^, ux,3/-|v<!^
u^jda
iaos»3,ixsja
ussdn^dsdcj. add di 12 ddrWu^ 14 I
nirt MtS xjdn^ Kdrs^a,
ZJU 53
acjprta
djsdesri;
.30 'd.'iqJo
*'
ri. .ostS
&31135, ay.d xoso
sc? aqsrurf adari
esadto3 asejuda
5«afi xoKd xsdd ebaou^
a
nacro
■LdjS."
rnvnoSjs
sadd.d
js>.
sdderiad}, tn xoZri
x^S>.
rodrc 00,3s
dar.ae? dar coadwrt
Ldjaoafi aaro^adoa erv.cxuai
rirSrt
Sriadjo^Ot), asciraijj
Bx’rriifriB
■Had 3<7£bdd:doa c:
njtx dors ddjdosjs 'Eddnsj, audex
wrooiici xicij&rasS
q X.^cdrt doidaxsd dux s^du,- 34
a?©ddsSdca aajavda. <3j?,l3 dx^r
asdavuadcds-txa soxccu, caa
34 aeiriaxrt dad svxfio, djxh 3iri
sjnqx
aidSriaoadoa
3riadx>«^,O3d End x,o, assridj.
a^aajbirrWrVs d«,rf ^o£<tx x/i
esriSj,
dBopadoa
svodjo
XdKds.o, di cadrt 34 ust^i
aajicjdodd 01 arid ddrd, aa_ adj,
udaadou oiiseud ndOej,«feiJjx.
dvaiy^ocsjdoa ovaxuaa. uoa
cs^caasjasr dcuj? odds uoasoa
darirt rjStxa> djsiy^dja w ur), aidrri
4,13 aroucsa. rioarwd} a^dda.
d>td udtatzt <70,
dddrivd}, xoxdcb as, artdo asos,
ixrt ctodn?, a&xddB as/^ri
ertdcb. tsrtass, asedoS uvri Sod
driOrt dawC oodxbd «X.S Xi^oo.
daoa rfjwa droxsoadda. odd tsodcix
a;a ux^SjaddJ, add audadd ©i
scoa, coda}, nasrt add asas,4
xjiS-dt? d.d. wx,3/ said}, dart
dsdrixb
dSddj,
aao^txhxda.
aoaa uoa 83.
e>rtddj£. -:oaa Sard. dxa. dat^
'e>o,a
rpaasr'
aoaa
ata. eaad. oodUSri
aodead^cod t-oa dta^orf caad
.
*
oourtesd. aoex. dodrf.
01 X04 8.CX05 aoa sodHodod
ddjaS t-odt ddd; doad aedjsoa.
Eddrwd}, 30o?ax ajaa- i&t^rartvs},
doddBVrts doa. dodd dodeix
djia a^Sfttfrt stfXxsa d^crc1 aoa
airepa. ea^ad, oiaj, arfca dxa
dJM naao. dadoysod xocadfi^aj,
dda sdcOd'aaM edu,dp wdj, aCKtiXdSd tjtf.jjo. dadc^aaa rtrd;-s"
aaricJjS. ■ eXSpd
eaadritfri ’ dado^acd Sdssid. rird;!?' dade^ada
djsoQcdah uad aa, «dxx4 s053*'
fyJasXd Sctodda. ajoo fySOttf
ad) ijsodcari i.rooa deS, Mddffiodri
aaoaoa sua ddea - daa
dtodod aoSfitf wso^ iitJj'jrtjM
ddrea xst^au5 aab, cV’‘4°r
cojo:d aa xdau. ao ddrtra dXOXd
4, jdJts rivaj, troaclioehx
fylosed
artorS ao.
ScSJSOXcja. d^ql.or r^KO' «Q, id.
1986d CtidO 21 Ood 25rfe ssoeioriv1
d^roOdrWQ asS en>dcli3fhXL>arad
ad, d.d. tiX,:3ja Sipsett CZjaridQ, 7
xooiod, ^a. daa ddcadssj, iioax
djstfirivo xsca. ajsstad iux
334a ddri aid dra aia^djiod? 0,
asa^doa nddopaj, 01 carid, dodarW eroded, reward ddrt ad
dijXcrha, dtjpojj, dode xad, soadj. od, 6a>xocadfwaj, *
004,4. !?.oS
25d djasri ^dd dis^aC- 45SnBj aed udddar}, djod cadis dad
e$a
01 ajsdddojpa Edd
rtjdedrt djaerida,
oidaa^iddtdoa asxcjcaa. «x,3a
tse}® adnr sad Od^ea
*
ax
a. trerax1. dodm^d 31 araa
erodciiserta^c&e
dedoa
Eddriva^ wjdcirNfwb^a deddoa
deeJdjdoddda tas1 noa djoe. aaa
atVid;' dca aSroed aradSXddb.
6054,04
d/TJOdrWd},
ai3
01 ana noxsa^, as? edood erodciBeriXuaad doddda 7j,X0ta.
Leri djsoddjjourivoo? doseX. av},
OSjyd wx_3a no, aarri«ao doua ada 'adod road, 14 aorj
Sanaa aca cased aJoseriOo, dosd. deiriw uCoinrideroa
.
*
airyd sCsdaxd aosdsjp 57 a>4
EdqS Xduroa dBdd stjs
fjXcxcS
vxicS>
X.^c/aod
o}Bdj5Fd sor), ddsdd rid5X},3d
4>U 54
oo ass-oouausad a3. na i98i
cpaaarucd d.d. «X,3a apadurKsod
ctjsrtd
asOj^.
a.
wo'.u.
toaSrJr
coudd ixSrt «ar), d4>q, dB3,d dra
adn «asa, di oxirt 18.000 dx>. na
Kfis ad araXSd^cSie eo,a punl
djXx), ;'s« rai;it! dv'drt dodd. di a.
ed’.a.
ebodrfr ^aoda
E=Jt;J
ocj)°
xasod XdXta.
XjdaJs?
Xddtf
dS.cJjw d^dudaode?
ai/ss as, oa ssoadi); uas,d. d.d.
tostada sart looses' Xdoosa
aaa^drij, too dodcx sddcja.
Ucdd<' aoad djjrfa oxsod 1984d
27
tidrt. 8x4 cda
ddrrWod di?, asoaddcsridd!
.
*
esde wdodafid, wo,ss ad~r drad,
Hodc? aotsa. odd x;d Srfaalicjdjs
'doaed ddesriv docarWrt wdt3' cou
dxoxfi, add,; edrod x^di^a. <34;
bo, dca dodaaod Xasro fcjX,Sa
Lllty EddrW 11% Hao asS Xduosa
easddeioa
aaa
"iddos
’doavdddrt tsdsjddij, eo,xs aasrt)
a. 33 ddfc Ear? xddcodw odsid x^a,
odd Juan adj Xduroa daaseda
45% Bad- 0i Soda Srdo 11% Edd
Xduroa asa^fjejd di xidd ai}/1w
ux,3 dataset ^dCo, tsdjB <psdnr
dadoad roaoana ^dra, udv ^d.
'idJo s^de ud doJjMri ad
sxidj
tsorWdi!}, dd aa, sjarWddd o?ac4orf
^dijsda aaqcja xddwj^ ^od?
worWd^UjjSjaca a^orf asaad.
du,Sja0 X,3: sEtl aa, EdqJ
anadddde douo ara^ood ed80
ddoioas "idCo, uoSaoSrs ordeJe ^O;
di
Oss
djjjX-dodrfcaQ"
SdSasAcJdddj, daa 34,XCS„; isi^di
wad aa, Edd aria "labJ (ap’.Ga).'
deddde os drac
*,
Ed# ddtin, aria^
na. add 2000 81W13 d.QactoriV Edqi
irodiSrt
ddaart
a:ad.
34,
aaddds^ sjxid- 2-40
*
mcod
wd.«r) d^dricaaj, edA-rr\5daridd‘Kd
Kaajpeias
xo^oaa.
^rodoJ)
aosyjprt Xosjicdr tsQad. wdjjrr^
J57i3! 3JI2Sjs;U)
aq». 29 a? 1988
dadUoS codes. odddj. adds
^ctS> nod aiij u^. djdx^dS
*
■ daodd so
* fJjoeadjsSfa" sod.
/----------<s5ot3e3s' rfda
'
*
(54
4ddod)
osoi/lvo dns, edddj aJ&Xtoeydb.
KSd odaer^ %S:li.n5fl doucddiBsAcj,
do^ tssjdjjer^d Ed? sciadSdS},
uuv'Xto- tiCrtoo 3;
* dtoQc£ Bo
*ortV
eart aste. rorooo^d^, 3500 Edtp
tScaarWd.
400 ddj, did dodSrW
dov'dj, 5t>x datad xr^ dodarWd. odd
3500 di^descs 10 dodarWQ SS,d; cd
aciyrt asdrwo.
SodartCrt ddroaA iacbd dadeo
dotsb So3d od) SoSsOAd SddrtVdj
doea/e». daco aorort Sro
*d
dddeo
disod *
oip t dtyoto. ttoSdrirt
*
di
uVodSJo, dioloocb 6oda.
Sodaad ddOeSdo. odds dodnossd
soosoyte. sgSo
*
rojf dacdd tp^snos
osdqdaj^ ddri, adds roe srortd
dadiisx odd I984d doSd Si
edsiCnt'j sdddj. Seda 10 SroxMrt
*.
Si ‘lessdod zj<? ^dad dado drodrai
oa^d oosjs u&e *
«yw sod. dadoris
dOi«eS- ea^dri- Jrtaod Sdort
*
‘■sdtyJrtS added ^dtdessd si ajSjSddJ,
tSsoadj.
etproortdo
abduorort-dadaodod
dooat^dod
wdtjpsrtd ortoucy od^drtdo. EddrW
. "’O dx 1988
cot!
cisa
I ^ca;
I 403d
dad
a»oi
roooi
irotse
19
*
25
dl},A
*ro
*jdjrws
£diAd'<».
05$ ddeddh soaoaosrtek
eosa <40da SoSd ded ddAoSad,
10035..
aKoaQ, Sdd jJoatoifidSj, Soiraon.
dtodea tart jjodsd Si djdddiSj,
<d0L!sf edeb 'd3sc sa^d' (dodro’ uau^)
aoOdjd.
ociraer^ort
Edd
Solrsoaea
dddsan- ddoaan jjodca ood. Sdb
tsirt rttj, ddtiijosj.eo dxOSdOrt ood
djstS>d)do- ss& TOdsssA
odda drtr
udd d503f(5> avrt djofA oddj, ddj.
dnajdsjds. uif.ca odrod loodd djoddo
eddd}, aSorta dKUJddjdo. uu^d
isdo
dsososiridd ^rfedo ododQ
dOd- dSrd^a sy^-S Xojj, e>pd,3 y
SrtdodisocSi
dtuo SoodJidjoodo
djodod SojJ, ^dd}. dd^djootd
aodddo ui^.^po, eaSiasocoo rodeo.
Zuodd daa}roo3 soortidd. odyr^
dboytoAd. d£»wj iraoj, ro. aOreart
2yd. zpsMJTOdoS. ood 3ods ddd
ayaa odwortsfc IroQj dradQ ty?, o
ot^tfjCb 05dtd odioerY 5*OSf5$».
deOrtd: adj. doo dsorb^cbd troroddri
ddaeo,, dart aedrtv toOoduodrt
■■^dde^d rodd Sad aboadfSise.
djoddojsir dcaa eddo
acj.dSJ?, ^ododjo add ucs>ertdddi>.
<^odo doromo^ rtds.d Si ooj.wrtd
it-vs
.
dada^, aoddo.
.'.ocotp
actsdda
ada itodo daedd ro
*
33, ajydS}.
jdaeA tfedi.
dduoddj, ydoxd. odixr^ dioi/Ws
OT&5Bd> iratjjdjd. oxiod dew.
spsdsrosoti aiportd daa)^ diUdo'
opmo. ayaa sSozD^ aejddj^
edjodySQiijj
tsddod
arodrt
ddedoyd. sBoa, edroQ Edd sodarW
c5/a' 3d dao&csAd.
^4/ odd a*
iira
odoitj, daod
ansrbyd aouoeb dai>6 adod. oindodd
ro6oJ>dxoir dodnf odd? dtddoi. 'i>od
drocd$,
ctacArtV
odviaayl
odrodAUs^ ^tyooJB doct>da>iart
oU^KcJcb.
1930-40S
dii!ot>Q
ododjwija<Q, odroEprtdd^ rtydrt a<y,
rt>oaS5oy£cb. z»d> ^odo so
* dncbdjd?
uodo arodros oolraeri *
rod
$
‘ os}/:.
oolraertrtvb oddj, oAdjueAdoira?
dn3 rfjas^sfe. !uod 1975 deje dd5e
dedd odjNr^ d„d4 d;Add:Sodo Ayo
*
dradto
ooiraert *io^cja>. o
tjodraertd ddd ^odJ dajsdrboroAd.
diddd uod5 dAdad Arbye, o added
Aspsd^aod sSdOcJd Sdb^SJ, 116 Edtprt^b
d»3. 'oiCbAdcJ. Siri dJjjd? 70.000 6J5„
ddj, Eatprtdj! waosdo Edcp dodartvsl
edirWdj aodoSjto) sd# ^ij.od. Xoddrd
^cod dddrJdabdjj ^w.'S'rtVo. d6d^
es^d^otoride ^^erodeab?
ojii 57
Drug Action
57, Soni Tejaswinagar,
DHARWAD- 580 002
(KARliATAKA)
to. rwa«TO» TOwraj
ts&®?n£
sJo.oood dnciidodrttf? ^tjd wood
distort rtatn/W d
3?oo az^ofc
ti^rt wod. d£d:S8 tf4,d?fiSdtfod
wr'w^g uSirt dooind tfwu wfy atod
as^mrvijs,?. add edd del u^andtj).
■■<addj w0ve da, "odjs? dou dd
..."
doda djOdrooa
^^daw d/sd dVrija
daertdoip doodd rtjstjjzid edsrt sS^
rt.afoKb, doti«A>d ^odtfs5' dcddoixd:
dt>35d rfodrtdjtco^s..
sadcra rtjsSTOo3
♦
cdj®^cS« sso&od rad's, «aTO(^c3«
tS^boS
S^S,
*a<3©
sSjdjdo »9«o
*
eh i^dxSo stodod
cradouare
toss’.
toss’ awaddg ^^d^c cktiO
* toss’rrah iSeatf rtfjrtod toshoSoua toss
*
d&J
atoxiorig eatijt toooto(3.
*2s.odo toss
*
tfjs&k.taa
tod*”
d
dooKraw
fijaenrttfe oido .
u
rijsdo^UjQdog vsO° sSjdaaNtdg &ot3dod «h toSs
riVo
*
x&RatoStiO?
ra4)rtV jniioSjaerttod ertod
uodo
«agcS.
»
•
3tQ, eawodod dtdd dtOd rwtidij
dJMO, radfSi, drad'dg dirtedwj. ‘daxri
rtjsddi’ dxxd ^drfddijsodo ^reodoJja
djVb dodVo. dsoi drddvod dark
ddfiia,. "uod> n^S; a;ort ddS trcsiiu,
dodi dd:d xfyd anJ tfoa fca^^ddsj’
dod> dyd^o d:cddja td 3i^ urrtOod
rfdoSg. “do/tfrt ixdo »sU, usa«i4 W;
raii,. dbjdsrt osirt; <no dw2. wo? do
S-oa, djiirt djsjA &B,aa" dedo rtjwrtdd^;.
csajjds BJSfy ddci> Sjsdodddrt adOg.
«
♦
♦
dafcyl aoddad aij, uddsn, odeiase
:xd> t
Oo, 'rda^dodcJ:? idouiasodV:.
"oSjxSjss aoda drtrt adjs,d, d-oddasR/i"
dodj> tfaartJjKd^j. aidsrtu «5 dradvid
Sjja, dud&Sj rnaortow, Jas^rt Xjx^a
dwrtdto. 003,03}? atodao; ddao3 dacS^i
3fod} dd dd>. ddrorta^de edVrt^ dao3,d
ddort. 9,rfdoi.'of 1986 sj.35 11
irododdd wcrt uoi^uociodo. tf,3xr;
XadiOAAroca edUo *
rt dtdxxd uod;
■JOd_ IroaSo, Seda &aSo3,croC. Uod
odRaeridroe d^cS^Cc uddodrol^ deeded
ado. doi.e.ca^ d c^d uCKro d.«nd were
aertdrt dro>:A iswoc dxd, dodairocane C.
noj iud; ex'aeddriddo drod,. node
Atfcironi:, dc,oirortC« (dCACg duo)
ci»ddt doSoieg Sjs?3. doedd drotSiXdg
rtroodrtVo. eLMrtCo SiodArooSdodoS
ndod EfoeudO irodo4' oee^rWo, drotd:
drooi Sddddo, noqQ Aa:^do3,dod,d.
ciieejdt e~,3,rt dro;n. troix1 i;c>;d drojA.
a?ox> uddo irodo^dad
noj iicro
Jrodrod, d.S.rtCo. Soda. doi, droidrtro
loewo4. a&d Ad d;-fodro tro:Ad. tnrou
Urodd dortoadroodo uoAeA ndo agd
djai. reross^rd uj^ ddotg cind iododo
iioU< ndeix; add doolie daadodu^
daje?dcai di tseS^rtC tort tiCciooS,d.
uinb'Tra :3:a$
du.3 A did add: d,d
d aid droertodert
V w
c^Ofs^j dJv°r(Cj I3a^Sooc5*
uVStf, sedCd. droerortCdodda. oiociroA
StJ OTdOdidds. *
6, ado^d airo^d;
fZirdj dowrt a^ded dwJnd u^ deVo3?,d.
nOddAbrt »dd ”00. add M^iftodd dsTOiA
awadoJos? add,rt ucaed EaQ uadde
ditfa.
neipdd
dodX^dJUJjbdffb A
d^d^do
adrot uedo s-wmJM, doe^nvdro^. doi,
auaojrrWdro,? irouo. iCoddosejS. asro
ddro Sroddi&od: ad Srodudoda. eqia:
a auaoFdnCo notCBidi aeda ^dtde riodd
udodo edse drosAodoe 'iroAa4 (Joed' aoda
rtedo ugduroudodo.
dridrWt- 34,8, U.a. d:&odje?riCg tseio
crodortC deject ^edood. dadoda drirdd
d-d^ dodoid doedo^tiod di tttWo
dvrtv darousoorouxOraa^ odd ddeddedde
aS ofcrdjso&rt d:dd dtaid «jjd.
“Udej^d dd/sci ded^ vod,do tsd-aen..
ffb,A i:Zf" dried abdCrdOsf ifcjS’cd’':
wisiddd^ dtj d 'dVdrfrtdjai so
difTdd" Tc^doFf: “di^d dd^A
Rios” acdo dddolisfcd
“adodw iwd.de sdjwrt. dVdrirtron"
dde^d
dltjd
*
• dod?, dddi-rrf?d‘ ridO^
a^Oioie dsde a5j(? - wdi^cde wiao5' da.
* asJoTOdertiiFie^ dee:G ueatljOdd
ud.«d dAdtoiAded dde, aou ipidrf
cddgdoijdo dwK. to? ura d disdericod.
add rideewioie ailoOideriCod Bdd
s$U 12
ddert,
9 ddowo8 1986
dideddde^ ?i.'edrt.’sid,iaood. n^KSdrie^
w^OtfiS.
aeaioa
and
** o Sjeodeeoiee.d
u
tin
ddiadgdeddbdd.1 cde? wd) d:««js«bv
doqi si. dooddoie? ndde^ deanegra eroded
eaero^sraAoioja dddidie de^ssrajCAoin
Aoiejs, s^ixd devaSeed S4 deeddedd
do;Scio.ro dcirodirta3ra,doi:??
nod oirodd? 8J.cioro wiwrdg doodj}.
ntod edro;-A6ddoK Cd?d?5 3odo4rodod
oirod,d: dde,ad
Ssddrt deeddjjcd
eAy. dojacds uwjtf sw^d eepr 'Wdodd^
SodaSroidod, tro^idd Srodod snoc^S
dioiotf ddo/' aoweogdus i3iy.oio$d wr? d
djd di 'drod icoiddj^ i(rod drodsrodda.
ndo deeded aod? Sueidodd drotAoie
tfcart
seSosjde;?. ado dsrtcdd.
•waeXddrt:
siiddo^ aS'J duo WeS»f
uooie dMitio, dd idd ffijsep'
edf SuSdd^ droddod. odd no^dofd
aSt^rt
dt}d dVdrfoloio^A do
darted aifcd edySuw e;>of'dot drooad
ddo^d nod,Oosrt aeiodede. sexs6d,d
d;C4 ndo. uoda ddod noJjdo^iig -di
aiod edouSro?
(d/,;td) ndod;do,
300 doCn^oddjj. «di uodo daa^ add
troejooiog 'idd ado dti^ £5dM<5» nd.
no^dadr dd ado, dodec trooiooio dd
ado? ‘dito ioOddi, d^d tiCdtirtcdjoA
aodaroddj'dcdd ado? urtaoiooidod
dorto nodded8, ioacra^ra qoa;dS doeixd
SroC^d?? etdao wii, aie.aoA tse^,
iedro iedro idrooeOj dCdd ud^ ‘^4,?
dVdrfrt’ acdo 3!xosoje,dcdo? nddo?
no^doErtg ndod ndd dSidCcdd OFfSa
dtdi diddaoiddjOduro lOdu^ erat,
ndod audos4' a, 25 au^ ore^ ndod
auoos4' a 12 dedOj acdo du^
ndod OdOas1 a6 ndu dja^ dx;0d ;■
a3, 3arod,d dorosTi droddro:rta8,d o'rod.
add dd aoscae^A nd^'ddro, Ga? fcda
cioddo jroioJx ixroiSo3ro,d.
weatrtCig ndod uodo udvo5o ddo_dodd
S^rsrood. add o'djRxSd iSrodd ndoddort
Knb4 aodro trod.ddy. adodd cia^d;
Weairtgcioro addl’d ndodd^
ndosjck n^. ao^e ou, dtdd dd^rtCo
oioc^daOj ao^,aroAdod,d. twaoddrtrt
xrooeipeoio iodaciodd ’Ada’' iJro&V
aofe4''-^^ aoatrasbrtvg ndda dtcdjeC^,
4do ado itro^? dortort loride iorttfro
dixrojd aria^dOod addrt iz^rsiodd
irodd artod,d. tredro dorttort rietddoAod
d'd^ iz^n dtio. add,? drtdoiroc, di
ueatr1 aedal aedd sfcrtdo dootoJrto3,do
dd^j seu^e - dodado 30 ddrtd oeuifi'd^jdof? drtdairotlo,3(diii5?
didS, da reeodd drodSoindd dJo^ACo
drodoioo^d. nd/W dd deu^ dXrtroocrort
** Tz^e Hz^tsddy
.a
rJ-jXjyzew
dwswf *53jsodd
sraey djzroc
*
3d
**. cy
W
*
endoddOA O334 120
*Crra,oaod 170 dzCnyod
,
*
3z^ssdo3
**. Tddy row w-a drtcfcijitf,
z&sdud ea
*
M3. 200ml.
d
*
300ml.
sort row &Mz3z3o. (eodd *
ra
&
eqSr roi£) ddc
*
drod zOQ. stxrado 3ortV
ddAa t®, sara SzMzireid 'rd
*
edy
"ao'-^ i&cXjyoosF dX>zM
*3o?
eyd
S' zy
*r,;-.<ay
zJ'vrAiyr.dW *rdxd<5
au
aw al eds433ncd 20 zte, z&u^ &
*
3,d. 'i
* d4dr, eczradd63.
Xeododofc.'
*wo3,d dwc8 z
*d
oz
z
*3
,d.
iJUasj!’ rioUo,cJ
Mawnvziy zSAdrfjstfyzj
*
* & *
d
«3
y
.
;
*
■wawricyd
dca
d
*
*
TAiyo
Arad,. *rt> SOoAba
dtododd 133M5 d«fc. rfrtod do^idd,
*4dj *
3^ *
odd U3,d sraJv{Oj
*rfcftSy
cteacrart d
rt, a
*
rt
*
ayorta e z^do
4do
*
*jj
dda. dVdcirtrt dadoddrod
Addjse?ra^J<-i35' ady, u^Sff’didoiod:
ay tiozSarWddja Sjsz^lraVyayd. edd
ruidjicspsrt cuf dtfdrirtrt 3zzao3j3 ao
d:
*
aCoiws md;-3jfty. 3ara_driMy 3;«dd
“iro^is
*"
Mysydl
usK®1 adijjodos!
tzzt&rtvi ad
*
zd;S3dado3,d. ac
* :
33,3. ts.a., A’GoJjsjrivg Sjdroft d,TOd
*
rte
adddy dea^doqizj, adddo&d)ddd
* 3jj
d
*
dJid,. ^oziW djiddg
*
ao
a?M
*^. iJUOosiW 3edd artn 3o d ^yd
8a^. edd ^4 djzdS ady ed33zizj ezy
dod 3<aj Sstxj dQidadj. ezrod, Edd
*nrOTA dt, Stdoo da
??
*
Stcn «dA3
*
do
sio, dod^dJ, edDO djseA
*
dwd-ra
ddosydddo.
“wZyL eAsffid. 36, ~oa«5? SjoS roS.’’
aods djajn Sftfrart d.d a?
d
*
TodW
di *
oart Sfdd. radjid4*
in M3W
al a6 ai2n
*
wziw djsadg ixado
S,d. ^d.- d22
*ddo^
dJ3d,0d0 dXDOtf X<3Aj
dd 3
rt
*
drteod ajdo- 3zot .3d
*
3,
*
2
djsd >j,4cdtdjs di Tod^rWge.
dfrid 3ed dosdaadd^. eodoJw
^jty d,3«,<
*<s5yoUodJ3a
tAoeOoi
xadrtjs sidadzdado
.
*
arorVy ^oqra '•tozSJssWo d,dd Bjadd
g Flasd. djwhc
*
o
*
doddg da
$.
*
djwo
3edcrart d.d
aa, tmt di.
U 0 Sjadadd
Qu
djaftradoa dod djd.wrt, “^dSod tjrfAra 1”
3rd Be
*
d,djwdg d*S
*80. cdwydr
Maw ejsdiodRddcs aarz£ djaeriri
*
*
ao
A
*
d
a 3add
*
zort. c3«
*d,d.
* & wwwy
d
*
arowrtv
cisrodcra
. o dwda
*
W w *38,r 0 edjz dr
3o3 Zo3y
*
3y z&Sy fcDitaJdS,.
*
'Mawoixyzad roW ac
*
ciried
roaia^d 'Tdd traded 3zde zid xM
** *
y M-yc
*
dodd
*
aa
aids'
rttfejy SeWSg 8drtjdcd4', djs^rig 3d
*
*d^4 io
*
*
ad
’ aAodddod,s5.
**
dJodeA
*a>3. zsax, Xzodda roAciiz
*od djzr-rbzydood ero
dy.
*
adds
zidi zdzSy A:-o
*dd
erocdroo. odd di
d
*
ezrat
wfy dreS, cto^jd? rowndwy
adodo^asj. d dt zSedozjag *
s.
rowA
* Syrox
*8,
36, dzfodd Bias4'
adddy dT^drtjpVddo 3odr^3oddo coodz3
SActaSjac, ao
**
zd 6 *
*
xz
od,. add
*Sy
S6,c
eroddgd TssysJic
*
3.ad
.
*
trad
* A rowrttfg rosdzd sds4(
a
rozT'd zradra eddy zfcdsd X.e. tree
rad *
*
si
3y uodo3
*3,d.
(AratAA edt-Ara
S3, aodod zpsdOTria
!)
**
. rowrtdg
adJ^eros? fttjfi ado dsrori
*
Szszk*
SJO ao
*
2o?«3ja0ov33,0d
*.
odd tatfi
jdcdd
dszjpli)
6
*
11 Sz8ro Bd-y;
roc? Tdod d,dddy 30ml.Sod 40ml.rfad1
*.
doaoitJessOe
(MS, 5ml. *
53, &M
*tfo aqtx
*
Kdjjii *
£3) *
frod
*
30-40nild
d,d
A'Ododd («n
dSyAdo atod d,
sradg
*
**
&da
eocraa, doaa. o^ti d, tfWrsW Tdo
* Sezjzo
d
*
AiazA r**
CA
erooiroo
djsdrow dvddrtA Sro
sao
*
*** ad
"dt 3drdj»eri r.dj»:d3 azoairttfriy
djsdidaSjddjs it. adort addtndd
iwa^rt^
i;M*ndotdr
. F-liJ /sClfGI) {■ 0!
57, Spni TijaiAor.ogor,
DHARWAD - 580 002
(KaHNATAKA)
aoa edro^d djzznrt. atryd duscA Se
*3?,
* djdt 6js*
&
3 j,?
*<, d d4 *6js
3ra,do
djszA 3js*
- ts'jif ci djjoyddodjs
zpiOTdaon ertj^d. -%d iddyd.ddo,
aF%ddm
*
’
*sd Arad Eb? ifcsartw djj&dod.
dcra djj
4d:d
*
.
*
3vdd
add edustr^d
ddosig ayOA'a Araed dais 3
.
*
z*>3 j»
ddy *ok3jj* 3,na Od
.
*
aerfd adddrt
3;cra odd Ldcd - 3ca, 3:d dod dazrtnc/A
drrod Ka!qrtt<Sy *
rod ^id3aradg> 3cd»o
xod^y. ^Sjyodd *
dd, essdd.s duradr
rtvdy dodo dodo doaosyd. e^f ee,
edr ud8,3 dXydcdo addo dozadod
*3J,t5.
3JZ
dozii, *szX drau d~ *Cc3x
S3,d. tiddly dodo
*
dradeo djzdzds^z
*
ddr
xfedjaji^jSri'jrt zeraagd xs^d ag
Ortra edjorrij X3u oirad roouraAdj^ira,;.
edd zfodarW; zsi
*cra3rtodo
3
*
*d
25z
d.osoxdd xbtet??irtodo d^odJiozjdo dad
ijtjOdi Zpiri 3JZ>i3ZI
d^3, t2.r>. drtio
*rriz!y
*rwod
zs>«<cra
*oad. ^dy s^ort ~;3r3 zszfeosid
d4d< ^d. **
"*
da
dvreod Sod
cdoddrt z~odf oeioiig anracinxdjn drt
d
*
3,
‘*
G3z? *
d?i3
d4
’ Si d4dS *
S’czdjd^c,. djd.dziy SiudJoAoAvy aAs.
aad trodortjodrwdy aza eacsy 3
* v
zxt^cdg zczMjuj* zAjsrrtosy,od
*.
^dd
*rtjvd.
•jv
SddJzirt doebvod dJdo row
adabcradoz^sosyd. cs, taddrt row.
dxrozto; row, dd
i»
*
row, rtzjfrf
odoAraodo row, addzSw5 eddbAraodo
row, cjd wdeo row, SzSy, Ar«:ttSo:
row, sy, zoosw row SosdA SAM 36,
dtfxSSjy row'!
Tod doddScd&d ,7o
* Ado^ *
8d
sdojzrt, S3, t* d4dS
z
*
*
3uzjzc
z^scz
3odMV 6dc*
g s*
6
Aeorb3,d. XiSncSJ'J
Sd 3zydy jraSz eSzcraodSSsyA djzd
Sowed.
•rdS^ TOdrs, ^adj ffz>a wsyjg <rgd
EzSd Sods (d,rf roSA). A, dzSF Aras,
zAode zozrOA adc^ TjOuxSCuooodz cbozy
,3,d.
*
As
(As *Ort e
* eoAeS^roftciwa
*dJ.)
uz
Soai 3oin
.
d
*
roi^odg zsse
ira^yzydsyn *
d *
d SosSirW z&osri
*
dditoiod { zie
*
^ro do
*
&g. zArax
Gdg dead *
ra?,
d
A
aSs *
rod
JSddA'k ***
3djz
*
^jrtj
dr?
erodes
BddAVAy zwSd Aoueg **
8odoiprade?
>
*
eg
'TrJ.ojy *
dd row
*
EdqW^g
ffcfc^grt 30A dn
zpodz?
**
•
3ictert’
9 3
8X^
*
1986
Z5)U13
t'ifJ-J AcliOi)
a 7, Soni Tejaswlnag^r
DHARWaD - 5&o GO;
roato&ritf daed, tsddd daed,
daaert&eadd daed
tsc^aFa^
Ed^d^a
aadrtad
edwad e>d,d,ex dadrdfiecsad daar£ dddod draSe^ad
enadeddod e>de3
ddaoad^cd Sdd ooddd^a rfdat oasLd sid^^e Z^da^d. tSeadd^d Saddd^a
zsrfdrt Ajrtdod daaa, sad^^ded tar^d csadra^e
daaftsJdjdd dd ^ua, e>de
;dodfd£o3aozjo3 e>t^dd dzsad dd^ ^adaa^d ded dodad
23d<d
tsdaddd d^fdddddod ddda ad dedod tsoaaaasa ^dB^ddrtaa ddo^d.
O aadna csa// rdaessau nawd
oin^sb iX csto^ni e>i,i?
o^osa 3cnv Led dcrWaaod
«a^
danoodrt tx» 6dd3 XoastaacsaSa.
edaosd ec^s. dsrts® edddj, wc$fM>
udd « d3d xsd L'flSa;
‘_
adae
d:3d
ssrtotfort
edaciBadotad
6t>d
EXdrWd^
tadoesywri d^ Xaadad Xdr33 ad^fi
ssdad. sdi5.od? Sd^dS),
Kda^dSdiJ,
‘Hatch Bill’ dow daxradciiaoda dtirrt
udOd.
ad;t?3nad EddriVSj, dtd
□aaJ/Wrt safu Xaadadida csdaoSd
fdrtOSO SM oqS:d3fSd ?io6:3daia;3a.
dcsaLdj. 'a^d® daatrad’ XdsSdQ
ecAteddJrico edsaS a:ad;3.__
® tpad&cd fsrtos
di daddrt *Lasa« odd}, Xdj, itas^ad
od3;cdda i^odjsorah csddj, deriv'd
ddasxd;3oda SddddO LdoaaieaahSa,
edaosd etas® xaoaa® da
.
*
dd^nfr
Sdacxxoa efijS wandarw m>nyJd4odb
udoad/Wrt Xaads EddrWd}, 3ttj,d Si
XXfiScaadj, 343«cda
daeyXia
3dcsX?Soda xayaitoASa,
eXadb. ^czjpa®,
d^da adara.
x^awoqs c^ats oaaj/WiJpS »xn? exx
dodarW dzpad dJyd xodd. ts^rW
iw xdaad^tfdj, c3sd ad>t dasJcda
assdw.'jvS.'s,
SasacdaSd.
dtiodd
adj^cbd otajjdt ixaji SaaoSa (sax®
Xdd®)
tscjSdJ
daao®
xaiaCoSadj.
^rtyjaocs djacu. d^oda djaaaola ddcda
Si xaaCcdfl, ^dadjda uadsato aitf/J
aeoo etpxa eeJja^rau® (dad,). Ldua
St^xartad
adoua
urtod
*
dsacddri
sartra
dudaa®
Xccdaiptt. ddi^j d?>o6 uadaadd
djasaodhoS duj/Jdfdo, eddfidad
dsaciad tnadoadcda aqjsa edoadoia
douadt artjsd-
ScdaOSa^d. wtJrW d,4 ■ uodjaodfl^
d^;dJ<. u^.cddja;. it^djat doda
^<Orts?aS>td- daatirwdasd lasd’ardd
dcJjNfu, fSjNcdd dcdJj, d^dcpaS ccarto
da,dradid dOd? uddccj, daad^od?
OcSstl <ja;d ddmodrWdj, dt^daac&d s
udjoa^tod aodartrt (aodd »f5:«
caj^VQSd^sreadsrt'.’iSj, sadtitiaoudad
*)
taodsavsaturt'
sSdaL ckdd JSdql
ac&odp
tsgiisaOrWd^.
^d6ddj?,.
□aaaoadrfrtsjfi/a aaai^rt aaaJSjaWj.didb Sajjd
dtasadsd?
uoda uaoaxdd diara : eastXds®
ddS, ^oda xttpddO oa^xarc^d.
3dwja?di
iirat^i rt>r:daxd;®>d Si
dj?3 sS3>c dcdiJ, wdtd urtad
*,
dJadSsaAd. ^ddod dS,d ac arrida,
saoSaad, <^3d dodi ctortrWrt dao
adrdid’d. adsdrWQ, esae®dssrt aodiae
ajdaa4d aadoafid. s!xataj^ xadtaas 3C6C
^cbd adeS Xcdtd asapddodan esaijd®
draoaudd?, adtQX Xdtsad aoda tia),
SjadOSiSb. add « ea^ BaoSarcUadd, aad&d
dradd!
Edd
BodarWa
BaoJjantSa^OaadeS.
xoda
3d
Sododdl^dd dasd a^sapd uo3a:
^d^oda 3d sa^otoocaood ddodatjpdaja
uo3a. "dirt daadaSt^odOdaX stoS/tda
tgarar aOuarrtadddri di Ed£ aaaoosaO,
^duaxab' doda Xdsad ^rfj^od: udr
XaadQXSa.
3olsaoxad 6odrarti.t>
'3Xa:ra' 3dat tnasa^drf a^d;S> xodaa
Xod daa(33a. si ’ddaera' xou oaca ctadari
aadaira;. ^£3 j^,c® odasart ajdarrta3.Q:—
ec3d
SXac d4dGda tla/Wrt
wsac^ds1 aitaX daaaadpt? ^dad. adjcJ
wO>3jm ^t^Xiat odadrtja rtaaipa, Xsj.cSa
dddoria. dtoa eorldrtja Si daad/tva
wood.
wr^,cJ aeob
d:Q. diXod (csixad) uxart X,dtda
aaddaijadaijX
'XasajdiXy'r'
eqSda
doudjadoija^dada'r'd sdcdxia wa^. ^da
BadaCcJ FjdrWss^ XjaXrtjadXaSpiodja.
'iddoxaA (xisddan dascrt) dpj, xa?od6
traouariaddodja dan^dtdrWC, ^datj,
adtt^uahd. ^edd® daSa. aaias® XesrWQ
Si xdcSa Ed^dCSj, Xo3rtda4ias!Bc3d
wddj, eresapidd s»ua- r^A sodded r-i3d
xta, EddrtVrtja taXaaaod aaasad^doda as!
^aatdtjdda. S soda safsaocia: z^ad3d
dndadi^rt daoa,da:Xi®r Xduoaaaa, a^x3a.
das^ipad^r 3otood taoSdaa. tsdd,
daaaod: enadctoAXad koiraed^o® -cu
draedadd troa^dfJasdi tpad3 £daad
XaaodaXcUoJaj ^d. e>da ajfd aOa^jd-o;
XxOdiS XtQcda Edd^aA dadOcdiljaj
tfia^ada^ addafisrtad rtav'rtdia. aa^iXrtrt.
na^cf'ri eidoj, rtaaa^de deCco. adort
dfrt rtaasafldssa? BXX Sodao±> dwaddcb
Xjaua MtS dad Ux®l3adah alesr’SCT’
kuBt±> ns^cX saa®a sroci Si^dilQ, tJd/d?
wna dVXia&4 daaddpda dfsp®^
SdcdDsad. a daastS ur^ e^Viod
AtdaaddsS?, aa^d z^xdsad.
uoud^ djild ro6odo® Jx-driCd^.
Xaodd sajdoddd^e. da® ZQjd^t dja^
drtod ^iraA^d&ry uudartiadccton
Sjauf, djaerCsad. udda aeBdiaed
daaddSde ddtd daiyt dusted.
acandd Si uaa^d}, Sotoo&d
dtddaeyddja tj3d3 ^doadddda Sdiwa
dtada^aode? deed dc<KO. ddat dead
Edtf tfodarttfc 60,000 urtod Bd^rWdj,
aida. 22 ddd® 1986
d;d 13
*
udsdj
*
«d
d
a
r uAddort d?p! Sddi
*hXu> di^rasod 'ict>d am xsra dda!/l«>
X5K>. odd ad> dXrt uodjaods atoi,
scfeoxdd cat? Zityd? odS0? o axd/1
GzlqlrW zijzdv s^olatrl ^<£>. zl&vriv' Oxe3. tioid ud
dad djiddUjy uasputat®. deXotl
dot®:® usSzSiJ/l 3o®>
*
rbraaarbd
ttiKrtamd^ odd4 Edt? osJd"'3^ vrtyzv,
odd de^aooaift d
*d
*orf eoddu,
SjxOdjNhodx s®d4® endwirtisyl. BCjj(
ero^lj ditjd dud as
*
o®43® Bait?.
taddm ddj®
*
dx>t®;jd:
rt»3?
uadro^c® dodart® 3o®so
*d
ar^d
x® *®.
£- u3xidaoc®A dsddr aris/i 50
'*
Ud.
d>d4®
xsato^diDsrt
"iot®
dndsstiodQdJd dctooato Edt?rW a^e
*dcs> 80 daijj Eddd® odnd
Oticdodexffl dtt? a^Xsd^ dorasafc
dsds^aeT aot® ddd d® 13 dot®
aaeaidO, add i.d odo®r^ Xo^c® 39
*
etpdwlXddj, uotdoy^ec®
uOicadd
XsCoid xo^cdd® aartts ’def od
*
3JU 14
sSxtl
I'ioi3df;»tdstor’ Xo^odd® adortd dnod
ddac®Q adoXuiad. z*tp
ad®tc?d
EddrWS ooUwasiiW dxjc&drWd^
*oXt®ddod> dd, odxxr^ X04 adraid
asacjjd^ dsx
.
*
"iot® d®®dt}jC®6®d
*. 65 dd^j Bdt?rW<2, "lot? oddd4d
dxioidrWsS}. adjdXoirtssd. & ddao®
dwd 546 uric® 6d»c driuc® BdtfrWQ,
tssrtjs 888 arte® *
w4 ddid adds,
atsjida5
d®3f(SO
odrt^aid
ds»MtW^ *OXoirtd. Xo£ias>3iS4ot®
Xt^daXeod o
d
*
Edt?rt® ied, arto®
e>c^ dOfsasd otouo dnc»d)da}yt oo,
dudoira add? dPo^od 16 duj,
dddjida d®®dt}.rt a®^.
tiraehrt .i&aiu&asri
oXrtdt dSagntWa^ BdqlriV^ *OXeo
d»'i)6 tndfadodd dra. usraododort ot?ds
x. ausdti
*
’- a
*ox odd ddodXj, 85 d/rt aoXsayl.
ujatr' odd odd, a- asosjo^, urtja
odjs4raor *0X535 >d. oddod fide?
Sodaod odjter^ BQeosA XdQiOXi^d, osjj.
djsjhrt djsXdjwrt.
dilj odjwr^ X040S di^odd), d;du 200
’eddji’ Bdt^rWd^ ddoXcoAdj, artyi
cdaajds csaortd ae, urtca datrtrW
aad/1
xjiS>. odd dat>c t*
ddQ
oddds, xsad tortod Edt?ritfd. aduSd^
djjd djsX *
wu r djsa daX artod
di»d tpsdclraouri adid
dX®
dXrWd^ daaujidjs^tx odrad datyj’rt
xdt?6? otjirid rdradiljjdsSj, ddid/tid®
afod aododpsyinort x;d6?
aod> dd odns6 Eddrio atraajpd
*
dOcSi^d ^tifyocfc dd. tpjddd adrivd
&d ud^tad atdddd BddrWrt A.“d
djjdd <4d. d®. dsddd/, arli/l
dij5ddtl%odi a»eo aa^dJKhrWcyl.
"id® ‘c^ajy
"
*
aou trod® dndodd^
ddraapsr *
dx
X
odd4 uod> ddrd,
drttxd
dijt
dsdo
a®
djjasKorWx^rtdcl. odd d^dk^odd),
des *kU. "it® x®d>
*
oddjad. cdxdot®
SsOdd 'odd}, 3odvdXJd;dood ddart
*
esd
"i^^il,' ad},35,d. doda atodid®.
d£® ttarld Edc?^ o^e. d^odsdStJa
ddjdf adc® ddrdd traa d.oadjwh
K>d
**
*®X
abide® ddo&dJi aJzyisj,
odd 80 od^ d^cddjielirtSrea 3c®ira®d
dousS ddjin adjj rtjid? dijedd d®,
xsdd djuadvd xioi®dd}j
not®
SeSdOji. 'XaJ, t "logos' '<4
*?,
aoaojpi.'
udOrt
dealadfalriS^,
ai-ayl
<®6xdjiod djid^l® x®^3.
ddr(S od®er\d0 djiddod "iot?
et^XrtV^uj, XOdQXdedod; I974dd$
'ss>Q Xioi'c®^ daxmod®. <dd>t
deddj^ot® Eal0 XoM oaiXdedoca
XiMrt X^dXuio®®. ao
*
ddrd<0 Eia
X®>3 Edddortdfjo djioodd usmes
o®tdi®
!?
*
ddac®^
a®.
*
wtsdroij/c® xo^fis anaaii, xacift
ipsdidc® i®Xfi tsdriJi Xdtno dodariw
deaidd- a
d®n tS^usld,- on^sad Edt?
*
SoSjOXco Xjad, cioeKXrtva^
admbx®.
an a®2jj ud^ ®ut?aiA xao®atd?
adjy atta "todaso sSdoadot® rorasd.
X®>3<® ddd dijseJrtiodic®®. f»t»„
Xitp. 22 U.'I*1986
aarrto cJosd xoasd
Has
assoass^ asus». si’? Ead^ djax&3
uo3t anas.'; starts udesrtto,
2320 slj_ boJObjO
bSJjO,
urttzc^ tzdesdri as,d
tzsorty&OdQ sodzuosz. ssQ XazSoSz M3
roddsS^ irt^lisod txon^ Xdasd dsj.
dsSd Ead dj^sc&gj, xzpzDxizsoBs:.
ueasA ’deO.s0K_ eMS adtM Sodartort?,
trotsyJrtrtjsOX i:3e 250 Edd XocaoS/W
3aao8rt e<S>Sz3 MU3z. eO/i ns//
t^Oset,
tSdztzcaodaOz aouizdz
usortatfxd aizsX Edd MicezoosA ao,
EddrtV UdoSzzs adijdiJCozt tzo3z.
rt a’asuo’dod dads djijtoz rtuso
Moddi. (szirt dsSj'.d Ot^ uadd. dzseri
zjodods szoaotzdz edS, Szaxzd
cjodSj?' ; diz^ d.-Sz?
ajOgq
djsdetJcaasa
*
usds
■ rartzs
azt.Lusaza’ ddd/WSj, zOOfuXzdidfd
at:,. ae,Oo3 Aa^ aosso sato drjd
ssdd5jt odiO dasdr Masz3^d.
azart «d=s S>"3t azrtzart Isas’ did.
dcz.dt aazrt. Isas’ edas duszas1
sod anaad aazc aotoa^j, odd
Iraod tA.'ousrtdzd)ca zssciz; Sto.
zciajdshdps. cisd escort (i-taias
*)
djsdd csAdcJ.ee oa^ zOOttzXdetfc
u>ZbO3ZZ ^dt^. «t-ZSO
*)ru
’ (did Ot.^Ort)
dsSfttfdz, Sjzto.tlsa. ero^zddodd e
effort cZdzz edad euzzddasdnd^
HdsSpx ss)t dtz^A MaxdeS
djsoira dad djiOddzCid; tAj.cizdz.
diftsrtd dd^oz adf wasd Mdf! urj,
drt.'s My-zd;dt
csazdd aza^ded.
edoz aoaca azs&jjav^dod Mdi
t.ssdoXtJiSz.
au}.d 'ess^ oAd' 'ssre
'xbsyid’ act: e~d. owsirtrtort/
rtaztort usds’ osabtsra etz, rtsau.
zaoa. dv’cdod dzSeVa dz^.-aksdzs?
ads’ XtdXdeSz art^ddjs dzsdSoaS.
r;oddrtV tzzj, usd; dsso Mdsd
asiMOsdzrtofd Sp^ at^cauj, rtdsaa.
«CjS
Sodd/tort
adds.
odd
uss/i^rtfrt
oQidrtoz! Ldosta
dto.
wtsj djdpjncy} aac djxArw
us?.8« SsSoSsaj, atjidasfi. cSdx.cdnn
djsddasu^d.
Eaqi
dezaoss
sodartos McS>d trjdzrtjsdrtvocsrt aabc
XZ-‘- 22 d.BS
*
1986
did^cs dza~Sa aooia a^doi
SjsjuSjs^,
Eaa Sodarto eSusdsSdsaccsn
acjSbdJScj^d ad/z.rt^ a^3 Zudrtsdd, sS
udrartsd, S-dirtsdidd^ ddrtt^oo Sodj
oSad ddja djsoS st£.ddx Sdssoart},
Sod
Scdd
wtJbdrs)
eciotrtrtdjs.
dazes
SZdirtozs
adsrr.zartzd. sx> dzozi^doeb ssziz
udfc dtdS dzsaSea sejasdeb. d>ri:a
‘dzuijse. e^pn’j ssre6.' dates 'ossa
dayiito Sea' (SnsscdiU ea’ cion1
^odsy. 'zzseoud dt? eS^xsoizzdfz1
ea» ^oacss' (a-tssw?). udzs,
*^
ds_3
abode. dzaaoszj.d' 'eJjxS stays
Soaps'., eddzastzsd’rf 'wyJcadd
3Sp so3s;dw t;-cdj. tjsditoi Ead
dsr: use (a-oss’), ^de so^rtd
dowodod 'djstssxy (Locost) sou
Ut!4< Eaa (TOSSdd
1983 dt,
ud.dzmd^
uods.
dazoss’ SoSjoa urj, tjsd^ca
ssriosdo,
aldsjsddtss.
tnz^dz
rtsrcsbij.d. uda^. tf£>b tided.
tzdssdK^ort 3;d edtS^ebd 60 urted
Ead Soodssrtva^ 'ids u-oayjsssej.
roiatra ad Krticiraoort udssdzd,
d^d/tded^ Rddcnddort tdsdash
^dz Eddrtort^ xdaowuz daaz3,d.
d.dt0rort Ead dortdoyszd adesdd
6o03z datoo Mdzdd. dartrdir, M_n
XoSdri. Eiaa/tort^ dQdbSzSf!.
SvsX
Locost, G.P.O. Box 134,
BARODA 390001.
rt jn’zauo’rtSf, uo.'zad<xsd-ut5/s0tzsotdzOvZdz, dzS4Vrt dzdtos atj. tzOXzdd.
so}.t). etrt Eadrtd tJd Sd>t
^dzdjdocnsft dzazF'drasp ezdeaedzs
trotLdzcdzsA stoedzuz edasdto, (dzZz^
sz.'-d dj^Kba 3«3f5jseSfJ aadjdart
udzdd: eo&ra gfasSa dz<?. staszio’
art
KjtosussrtzddJ.
taonjdzddt Eaddy tzsizosdz aariza
rtzradzQ. sat^ez dadzd s^szas ^d.
ae, tautrtv ziztei zlaS/to dotd
uorsseczst!,; dttze? tzdUdtJtSotzzdz
BesydzasAd.
as
Eddtzod
dddjsedudzczd «d. aoKsdsrtVrt},
dzu«? zizzt! d»iJXdEtasrtz3.d.
Ld:&
SodMWrtj,
tZzadduj.csrt
edoOd Xdosdd tpstx add tzson;, Xdosdd
dzed uosz. d;bt soaZzrtyjs. Ead
ecrtnrtV ^zstSdzz. a^a^djo dt dzsX
M3CU3},
d.Qddjsdoo
SuousA
LdtiOxcas. xoszc! od ttrf^Oe. ®*rt
3,3tca tzrtj/1; txorsv.dtdd Ead »so3
dadocansAd.
cijad
- dtddd s3.Srtcloirt Ead Dzbrt zS3aziot
tiidsy szotzzd csz^ sdesz aardV uod
asjOodascaao. mJ; _tcd> Edd
dzcdOcZzddz '■toddfiodz djsS M3rt
3Vdiz<z;srtt:tL ddtzcinodrt^ odx^.
198406 dsusdoz. tABLAdoS. CCS; Ead
SodortOa ’idcT}, suzzaA td.«Qscjd).
dto&ccz Ead daozst}. 3dze aaatzod
xuertasJ.doS az3.'5,sizc 'xiss,' czz^
riZsX'tadOd'.dZj. L3Z^d0d0. 3’XSttZSej^tS
L.3QA djssosazd a.cdoXtas xo^rtda.
aju 15
■suoa n « n 1 n buss lEjaui'llltll. IBM
Arb : BstQrid asi tizbratiQ
andjitSa s^Xo^rWjj sjsos di AM
sxsazQ abos'Sexdono tied atoad;J. Ead
troQdoqbdj, ^3d (des^, ertdui, an£3
«d=» aa?) {roadsrw aart dOrtrix.
e^rte
ti5CK_d8a;
3i
zoodoiuo
08, ^dJuXesfidcdx djodda djxaainX^
USkVi LxXsxficcd ^satjocb it>4 zJed
aossdsb aejs, ridsXd, 3odo3odoci>
KEiaso Xo^ri
*
n.Qoa~.
eodrjoa
^cacjwCJrta
Ea^
pzoozb
aoa-x.asdritf uij, ddr xdossdde ^d.
txJcsisd,- o$??b3, oodx> & ddr
eosssssh Eaa iziobib xoxa^o,
xboaad xdd wdad. nd84od2 xdtndaod
ddsasd issue xaoirt uabcx^, job
Lduoss asrtjs od, ioscshdsa xoXs
*
XdXtde edjSoslicy!. ode adotorX.
zadjfad Edd enltjobab uij, Xah„
Xdsodd: abessoto d®od iaecb^codids
aideiz Sodarw dz^ddfjj, daaoaxsdd.
1950045, ifiddc^c od soaaoiaocb
8eo32di. ebd d»srt84 abcb, sotooxts
xa^cboss. od8 sjaayc: izcad udoo. ode
Eddddj, adds dlij. 3ctoOXeo adeie
8od»c3x>od84 Xdoid effiaii seas. <^de
0‘3 addeoodJd 'oded.wJ^' aou Ead
dciaoxes u2O3eoS5 8odboii2odd ai
ades-f soda o^^ped Xdxs^dj, 33ri dewd
Sw.dXodXb 73a. aarrttfodojo 3iJ
dr. 3
d;dhode5t
soxdjsi^d.^cd
^3j$,ocb artobs ssdj. EddriVXj, ade£e
Sodbride dotoOXO: Sjode . 38, edij,
*
dJd&o
dXdwortVSj^. 3o3d,aXJ?,
U^XC^d ddjy 32X00533, odd ozj sad
atoc&d>cb 33ou oddrad. zjodasdSja,
oddFSj, edooax. 3o3uy88j24 odd^t
Sod. iraXrt du^atodf^
ddd
□boiddesitsom tao&exb 3drW cbdr 4d.
Zutrories o&jci asdradoasdo X,2},de ^d.
1978C1©, diOrt 3dC3d X3,IjSS Edd MiO35
drod uadoM^, job dedarwrt
d3d3d8), otraXosh edejd Iraddjaddo
aodao, odd So3dd add: ddrrivd), Xsb.
Xdajd
ato ade&e dodarWrt
ssujsdes Bdsid aedS2. e3 ade&e Ead
Xo^rtsJs uOodo^d. ^?3 oadd Xdsjdd
xs^ddj ^d2d
aou X04
dJMriri^asnd. ^32 trosyoXid Eddrtort.
dnd/wrt hos4
^dcd sjjfayd io;
Xdsjdd doue> ■wd? ^ex xsod Xjsd
dedijsodb Sd'.tScdd d, 8odart Xdosode
Seddtojd
tojj
dra
djaanodrtda^ 8$?d atoi^r I984dddrt
^do 141 8jstU dJDasoo aaa esbd^xdo,
esXoddaJp
3orts)x> Xa^d d323_ du^ajd:
<4d.
dsd&od Eadd
*
SVa3 3So.
Od>f5>d8jd3d deed, Ead XoiraedX
Xo^osdcb e>&8 dx>X EadrWX?,
xejaaxed,
trades
rt2r2d2Q.dEsnd.
trjcsddrirt rtrou1 aou XXjdod
1986
drtoicad ‘rbrtsti&af'
wuaort
3085232020 Edd xoixdj dftoridasl^d.
cOdCi abdjfi 'Xoutu25i^xc’ aoi»d2
adsje abqpod ads du^ duj,
3085232820 Bfid^ vQ 3 ooddrWod 3X>8
dOUOdb
X2d532nd.
rtdradTXd^
■XoqxrajaSs5' Edd.
djoertdrfja,
wOdoj/luaiicsAd. uiJsnuo djoeridS},
asdodd 3o3de$t 33 dt^uo, aio5 82202
xqpd. odd
HdeygrtVdj, dod
ddratadQ wa^ux atod28t}/1 Xducxoa
draddt8odd
Edd
Ooa;jrt«>8k
d-raddjiiridesx tad^cai soaarWrt ero^do
3o3ayiao, i-deiz Oodarwrt ato!»3
wsadd odd de d2d«os>orte X4ae3ou
dddX <70,
adx2dn36d
8132X8, aoiira udde
rf;ddGd&
3daJx>3
c4od
adjy
O8t>dj2{r0rW eosdo 3O8S>d5 atod
izaaatodjX dxd? OOrodd. EddrtVod
sSeddy &3X28W33, aft 022^0
*6,
usd
0433 232d2ri9t> odoosd j5,e3 ddXaod
ZuBdo da$2t adoc dXcdjoddjo, as^axdodal.
^dd aodoios ^od; Xd5c X32t aoXdcb.
XoX3ff XdXot». 82U20U ddod2. Edd
oortaoS
ddjSf^aaA di urtod
dzwarioaQ iflltrtmhcbs^d. aoesiab: 5Xrt
83,0. eoj/ OO, 8d2c3 83B0S> BiUd adit
abjstJr dj2tri80 ud>atortU2d5d2; 52025
OUcdsdodo
xfcts^dscd, ssdxJsodd.
as-drosedo 2JUd5 &35dodrod Xjocb
3jk<323 d2orid;ron ududSd5. deidadd
dado ortdoeb Xtaxd codraaocaA
Xodsod dddjwtizjdbcb. atlrae dddeeb^
Lad dud Xabc abrfoddj d^Xaubdo.
Edtdrtd uzj di 03258 xdauafi, aod
djbsddd ds?ad aodd. 3adad2 dsddoddja
‘'4oudj5s' djjajjdu no8. ~ aoeb wd?
uaca dsdodfd. uoeb djx-nd aaodb
abid, tdbdj^dj, absWXo, uddodxidja
dd.de dsiaabuorbdyJ. 3d6ortc8 ebuao
doo, iraddedoeb. odeSdodja *
33 Edd
eoriart abaeA ooauohaUo5. eo33aO3cd
Ead
zOOeaX23j,d.
adry
urtob
djiertrtdsb sajfc anx dnaijastyS 36, Xabt
ded^,d aou XdV XodSAod. dj280 ac^.d
U2t8, jdf and aboddeiou edud
ts^SdXj, ddbrxoa Xdobo ddjde
duyiojd.
dedd-iatfiJ, bXrtrde 333281x4, XozJedX
20232X1103 Eddd oonarwe, akoxq,
urj.d
ddrtdf
eroSdbdou
at^ab
ab4/bd8J, uabro^ job Ead oosxrWe
uababtyl 02draas2Ad. eh dd2 335X2,
db5X5d8j2oacbdabd addP Xd5d EdddV
d2Xt3d dly^e,
SjU 17
A
lUdiumiHilL^UI im'iwMuii
ISSMhaHIk'KtiiiMiCiilll'illllVfcd
W Action fof^frnainjidiiii £57)11 rtJ3$o57)Os' C57)WCo
57, Soni lej.'jsvirinagar,
DHARWAD - 58Q 002
sradcs’s rtjaessTJO6'
(KARNATAKA)
^cd4a ^>a),aod
i c”> d,d.dSrt
t) 0 M daaen
D/0. s3Jd4do adc^d^ dOe^oaa,d.
d^^Ovdo, Stdosa^d. otoSo
otd rfjtea Se^podi Eddddda^
wddoijaua, oinOTri drtdosijaV deifo
<aodo des? i^Afiaadasa.d. ts Edd
ridd/ i)Oeax>, J/e, drtdogjaoda
aow daada^ daertos/o. ''sS/daa
Z3o3 ^o, rfc~OTOado3.Cj.
OT o! SjO, ddOSOd. OdOj saaSaan
■dauridea. aeaeri Saaoda «od Edd
atdodas?aaodod riaco daadDdaadoda.
ud£>rt Sood^dda otad daad c^?3te?3jae
daaodd dadaOTrtoaa aadado. esdaaa
KedS/ esaoio ddwe EdddP tscrafid
Wuiodi.
dertodd et^odarOTric^dcdoed ?
a?>OT. aed) ^Dtd Staxid dJJd,
DdfiridO adtdnaAd^
zpudd^
woDdwdodo.
2psddde.'js aded
otaoSot
dosdo^djOdoO
Sjrtda.d
OTdoda. eca d.d.ort
rtras-fifn
odt ?
0 a
cuu3^c3
rijS^dOe^jQO. rJ_,d? tJcoFSD
dd, ddadod d a4orto ssFfo Sjsdo
.3,dad EdQsto aa djsfoo rtjs3,oada
nod ddart adort rtja3,de» srad6d ?
isoeS, OfOt.
0
V]
oujsddejirtog essraois
saO
oiocio
aandorf
5Ss3§rteb
desW©
iTOci®
ssecsa
esoriftri'doja
^jacS
£3 03 <d 553 h
j3js d odoo
53.
H351rorid
d
O30Oio Qjodod
ri'd?^
K?$
d w
jjsSs^rtKle^d
jddTOd ef>5Sd ftfdjsert.cS
zlj3«! a3o3 eJ cdcSXjcS.
eo
aoda tSSj en)OT3>dtS deSodd dd
fJaaed) doDrtoda Xirtod eOTe^a1',
edsra djadjfSjafDd djad, ucrae^a1'.
dajaod: aode otsA Stod ds daad^
risd dtddtd SodarWod Dad d^da
rttfg djacraUOTrta^d.
edaoaa,
,
*
a^da
^ozl/csWe^e aed, esiafa
sad Edddoda adeQdOTAdod ^da
vr
Sda g daad^
eorttarti'exra
ns/c-js
djad<do:S,d.
wdaboaa Sdccjdaadcd
Stodd
c<aoran
doad? dCajad
ddae
dCsCO
diriodaa pacadcsan daadctfdjCroC
wd4,d.
cgsdFrt,x toe!
e^rw^e
•
daad&c ojC do .
edda^
uKu dood
adeo/a^^
S0Cd~O CjOCOj ^dd cuCV'C. edd
Kridde ds^rtoreadodea^dt^dwaorra,,
t/eoaarttfeja ncrrta^ deddodda^d.
ae<~ edaaen.t> so/4a daad essaO sf
cfd doadd
e de^rivg
r->
<Jos
fuvrt dOCld.
tortdO
<5.a:!rt ■. > .
dartuaa Sdfd. daadwadd?
•■ ed: ccissocs ’u>de aezie aocb
CaodC Send dad. L'iC cowrij e o d/ci Fi d'csa^' a dj? sj?a iS^ftS,
cB^rtoi, edaS.af
dcaoSsartd uafccg ? 1 '
C/iFdO ■ eErae^FSd^ ' adeQS
cjdoda. ddo^ add do,df ded,
tftSodo ads? asa^ois woCda.
^da^d atddaaddedn^aa ded, dd
asa,oiSd dc^d sdaad esac^a6'
rto d;d, djrtdanart ede circs,
^COZCJD
cjS2?Cj
jijsdSz
ZuJvCcGcZzCjO
‘
cuCLcO
u '
wrtsjo
«*■>
Sustv>
d
b
Xj^cJ5
Cjjd-ieoi ijKa^a esacpadra z^rlSafosS:^ sa^SoS.dacJCjd
^jenan ^odo ea d^caO dtorraraosaSS Edyrtto uoSoca, ^d.
^odd SssjQriv SOoisad ovEJoSton dtonccJaw^ dasad, ede end
dcdodcdtoriOcd 3raJreiuaaoi'.ra Saad ertwodo.
JsdfJrW SaanaU
□cartas d,o3toririv aoiod,Ea SOcOjart ^ejdd d eodd dedrt^C
ac&!uo3toL3af EajQritf dodcdcdaaert zadcnan rfdota^d. -a^djae
doJSUa<'’ eqina
‘Sa^?daaeda^^woae1’ dxos eoUdoSjaeda'1
Bdipcda eacjjoSjS ercdaJjatrtde cadSaaiodo ti.wod erocaaddii.
‘fo> edtodo.sUw5,’ a-oda ert d odd ed.t s^caCocjad
daajnaracsadS EaSQ. d ^aoSaca5’ a^dd djaenaraartd Sdad, di EdQos;
aS,c3jacrt edot^aaaoiaSo. ddsa., daaa arts?/ di SdQcdo drt ctodde
soctod aed^oido, ■ ec^Ood eddo d< Kd^cdoda, cede ied'
rtozped S^daadd dtortnert daad, icdv od Oocscod wste^diSj
rted djadd^da. edd ^odo ‘SjaiedjaedaJ?jt!wf’ end sada^r;
aOrsado ccartja ei arid
eazordo eotficiortci
n ddciconan d,d.dod
do
^rf. daaado _ daeajOriKSod dorie/dS t^d ^daddOrt, ed^
daCeOoircdwOj w<dao3,/oc^da esadjdataartrfo, edda^ i^^aolocd and
node acrtda di Cddoirt^ aedoarto^d. ngoaoddrt nodd edaaeditdo
erosa rt
dxaadidda, £,d;ero u Bdt? SoliaOS
C-jjcjj
oCida djidj'3, daO^A
tiiJXdd
dfScda
^oSdo, ediJdjaVri
dcda aoda rtdOjaf^eSaa acraadCo.
dOreadaaaA SodnAVa
esaosf
dorjiodcdiw.^ Agddo. eca aaada
Sd.rt ad:ddaa aotrarid'O. uodc
d,sa6A end, odd daadva oq^3
Sdd aortartvg daad, daadod;i,dQ
t!dd tf,do;K> d<? d9vrWoja do ^u,
eortarWojs
dd
*
Qd
*?
caa
aatiXisSaa^oadctp^Sa.
*3vcodaa
doo
eAdto d
Td.-a djido dtaarrtdftfo!
dcccooadjd eaad dado,
ddae
Sd#
aaadcioa 3dafcg esaO,^ aaiosa dg
Sd^rt^daa, SjfeQftSa.
daadsJe ad:
oaa^eoaa siodarWa tfjwdr&od sd
oiJJd^
ded
dOAid^i.
caa,ridg
ddOTJLJ Crts^dCc^dj
doda add iJjctf.
dfd ?
d;rraA e xdaadd
dSj d.cda^iga stosg Sdtsdjaeoaada.
ora.ud Sadaaort; esaO.s’w daads
riarerid art dodjae^tSciaaoSaga. ^da
ddvCJ&ud^ OUvjg Q/ 1 JjWO. OvdJ rJ ,dj3t riZs1
dca 'j iOjodo^cdodjodod.d.
di,dO
jJGcdodtfjgrartw
deeded
<^dd
j^Scdodo. dfodo d<S_SdnV: dtddd:^
ddca^L^rdd
S _
dvSCCjj
rto^njtfc&e <wd ssd^dra
dji^de ^3: doaenodt &
SdQodid^ drtdoidrad^dd^d d:i£ri 3i EdQxo S.b3jteri ^od;
aVScj.Cd.
o.t• fdjsedo.xiJsF c?dc d,sio3:a=' djajnoraori'Jrt ajs3,ao
djoeroraorWS^ fraaSdado^dijOj &»d ^ddo, wvsed ra^asAcosJdo
Tiud djdvdoao?e^-‘3jojodo codd'^joAd( a_d
60dcsAvcd
woasAaja o.ad dod doood CjjjfzWrf ds SdQdo^ ^odo
A? duaAo-S^d.
add ojaied>raedo.;)da='rj djOiraertOod etvowsArd docSjOrsadartdo
itd rtcdjd djd.add^. djadeSoioda eodd, dod, dod,
SdQ
oioSo, tru2d,'acA5:d)dO°d ^Sj d qaejod5' daaerracaaAvt 3s GdQA
aA Sjaoda d;;cd Soa edja?do,XiUwfA S a.dp urt d djaenaraaAVo
deddg e/vouaA dtJOjoiddo3,d. ^o3d djafnacaort^ crad^ ded
cJjad SdQcsoja
aodScda dod,Oread:dodd - £>jd,3dd
d^dedd (oisaiJijBJ o3ea:cisa) trooaartaS.d.
ds EdQcda d,zpad
dodo A do, dcuo^odadjv dad ex^d-d, 3?5^ sacoaPdeja, ejdJdos.Cj.
radj -d do’Ss’XjWd’ oajaais^’Sod «acuartad,d.
doreadaaaA
dfddg wC^o, traoaada^d, asarija d:dd daajdddjafc^tj do,
Saad oacD3a3arfa3,d,
ofot
i)l 11^ ActiO
*l
57,
i 01 b!ii"^(ifiiot(i:'.’>j
Soni Tejaswinagar,
DHARWAD - 560 OQ2
(KAKNATAKA)
‘akroddaa SO, KoXoaSJoirario’ - '■ado 'aodo S/nra tsrts,dg
St3o3;e en drfda tado3,d. esdd 3, t^aacdri saodOTOtoi
C33doSoOt tpaddc^t toti^dcxxjojdd,0 dot^,rt tSsjaOo-ti300ij3hd.
'■aoOd i/datfarWa, d^Sdd cartas Sot s i/nadorttfo Saad,
ddojj ddau SoaJ.cdoO
Sdort SdFdVS
6 M Bdtprtd SOTtSadod
m
m
S^FSotjdd, sat^d? °;j> doda SoKi£/ato.4,OTot5. ^qSrofcg aatj
Sydedtio dodo uOTatasao SziQrtsfeb, ta^Sa^m^d. 'aodd
uroafcsao ;3,dd3,otood daad ^dad 6_,eOT^rido <^dda_, 3?d Sash.
•esttaafiaf Auf traced^ ’rtdo (Anabolic steroids),
‘aaaiti^’
(Cocaine),
*ddo s'di3aaosr’rWo
(Amphetamines) - daoosad
Aaot^Sj^ aaJjd Bdtpri^dq, Fidaddrt
*_>eraa
‘ooddoad^oX dsa^s
S05?’ (International Amateur Athletic Federation)
d^awoQsd.
•s^eoaoSo^’Sa^ drtdoSaoVa^dtdood wa oJoortd dvdrirtoinn
da~“ xartaa i?adodo Fd dW dQ OjJortad^rf dodo dt3U Ft aaea
d.ctsadarlsi'aa
m
djserirts?ori doslod
tfroriVo ^de
CJfujcv) ?jt) cjS'OOC
co
M j
dcddod : GlraEij njodoja
ctori
rWuT) deojci Ej:e<d cjts? sijswzjidcdi.
cJcooCjO
co
esjioiad ^OSdVc
ajaraoadododo
fadct^dccahojaja
djadcif^ojofi Setraau srtod Edtp
tssrafl 5? aodt eo. rqod ^Sjsh odsfi
SSddrWd. ercOTtddtSrt SortSdg ritpF
cjo^dcdor <taoda CjOe^Sod caSt'8
Sooixd.ri'tb. OTRsadS OTdjaeFrfo
ri^wja<|sirtxod w Sooioo^ridti^
ridFtSaiaOri, eddoja djado dojada
ioridO 3-ra^jd «t3o sfcrtodrt otua
eOTajosaO. ri^F drtSOSaa,, Sd ^de
Ed^SSa^ erodcJjaenSaaa^d. -dsri
Sotu t\ do
d.d,
draeohe
m
ej i Oodoaa
OTUjdarWodoaa
erodaJjafhSo.dad
fs, a>, ^aet3fF aeua esOTtdasaO
M
.
...,
Edcpdcdo d,d
6t> dteaT1 arteod Sodois
iedtaan djdjacjdOThd. rit^F- driS
eSodo ^ddoH OTsiaJjaeASddea odo SeS
djado^cj^od djadddad Sodt^Frii’e
Sdo . EdtpOod uOTOTh «ro$ai>od
darts add oUjSiOfaadartQod Saad,
d&jSSjat^eartde d*?05asd,d.
sada
SaoAd ddd d,djarad eoaod
saoJo, adaa otS daatdd Soda ^a^dx
oOTcJad j3d<?d®o3or tadotfasa^d.
Ss^rtvg r,dod tpaafohf ^c^a
rteriodaa rt$F driSod Sod 3toa
caidddnadoda. ‘dodo rsctit(iaf Sori,
dd doo, eOsaC
*
dodo aSortSdsa^
Sotos 00S daadod ^ddo e
daado riocarte tart, S,d: eode
wd dedoSadori ado de^cJaado ?
□,o3jae6Jsaes' dotaada ^SjaHoda
daadd Ed^t. ^adwa^ St do d, toaot
trosa« d
sStsbaa^d.
dtVodod,
crood
odaaaSd^F’ d,% Oded
d,roOtfaa,e
“^eoacdo^ ed6od doraadanad; dado, uda oua„
a/raatfartwtaa rtaada,.”
dood «,eda d^da trorkra dddeinadd tatpnajOiadod
“dMaJa" (Human Growth Hormone) 53aiQodoo ‘^eoatdoy
Aodexra
Sudo
doraadaeao tsariaa ^cd wVSoida-
Saad ^aot;Jae.
dd,d;rada«3)doa
^So, Sexj) 6,edaVarido tsud de<M zradaqtf-
a^C^ron aortas dtiaaridod ^doa Saaoes1 tfjad 3rtdatfjado.sa,<3.
‘^etrocdo^’ icariaa tsadaaers
*
BdQrte udicOaod riodddOjdoaatJ,
iiod asariaa CdofrWa sravariod sat^d eQo.
a^rJaa/ri, aaerajord,
dooortaaed, eadatooaiis, daadri adcda dtfdrfrtohdja, efaad dd dad,d.
doriSdg eda
daadrt doa£> rcariaa odoiag fiaadeartd
dddriri, riddat^Ood;tioaH Saad ercoUadJodad,d. ursodd EOQrW
trodoJaaeriOod x/cdada dda& ^e^dtS^e StfdaSaadav30,d” - iaoda C
doeOua6oasi “oao.eoOo Edd dadadoJaaert SoOd rkrf ” (National
Institute on Drug Abuse)^a d. da^o
*
ssae^ edda dcdasa.d.
i^eeooa, daelfSoiaartd^e adei?
daadc^d. asaraawg co,COO daoAd^
tsortdSodssa^n daa&d ieic ds daad.
cdada. add tpadddg daad, ipaoad
dan daauaddariodjd.
asdEjtd
dodnd!; adoiGc odaad fcetro rra.n
r$odi>
U
otiadde ^dda, eoddoad^oda daada
tfi^oaaod &odr1doi5aatfvdeJoCid,daa
eassS) daad, laodda, Dd tadO doda
«ja^d,<dt ^c3ocd.
ds saojadcdada^ oVdtJSad e>Qsad
wdvdda ‘apadiecda Edt? wcdaod,Ci>
*
artda
doda, edd oas^ Ed^ naaSrid
ddd da. ^ddartva B d $5 d riots
doiijdda, tJtXXA odd ed^dorsada,
cOT^BadS rtaris1 tart dDetJdoddo.
tfaaSoia staroia ^dddaddaa a^cdriad
doadua ^dda 'sdaa, uodddo ■TuaoJ
riVds, &%|d?oariod,d.
Sd#
deddg Ed^ddaj dodad dado,
dd sriddoadad eQsad tros^dda
ddao/fioiao dada, oatsa ooa R
dSa,rid daod,. daood aodra Bdqid
riaradorg daaduo Edt^ aajaodjWa
Sridadda, Srtfad dtSo^ odaaert
^eaalodada. ^da dd,^.
dada,
saojodcda d|«rad
4evJadaU.d docdaad.rteda, daadaddda
dtddO
co acoo daa.x5 ddotd Bdd
T doda
ada ddrrid tfora aiaJrt udnartadda.
ri<?d.
easJasfia d,di! oda cJja.SS
6 co
6
StfleQd Edtfrtda ^daa^oda d,daatt>
dS daadatSt^rt woda aedua sat^
daad^aadad ddao cadaada d,daj
6 a>
Cjjaddaa d°nd daardaarra.
CfiZdg
ora.
asaocdag
aaod
ddcdoad,dote;dtdo EJaaedaaeta. dda^
W.,000 daa^ dda^ Sootod,rWa
djoosuairtoi.d. udd
aaJoodjtiia^dodddo LOO d:cd qzF,
3jSjos'riVo dood,. rfood. dozc£ Bdt?
BcdarWO ? aedz M^oOrtfdod
w aojccSjSraQTOOrttfg ? ddO
(doOdod aod, acioo^rsapwOoEodA
^dz eSujooiodaS.
rtododOod
dUjo^sOoioo dodo,
cozooojoM doo^rWo ed«o aioi!, dzi
SriOddriuo zsnozoj^ddo. djocraua^
doosca edvd; djidojzdo
dddo draao^o dooajrttb. odjazdj
^coodojo aosdodd eoto. zsd^id
rioradOzo dooctoo Edtf acioo^trziQ
SOOCjOdO^ SjOoJO^O 4do dJO^j.
EaSrtvzfc
adzQSod
e^aod
t!ecd,d dOd eipBDOoioco dxd,.
Td.^cdo dzddzd ^coodriVo dcdz
crad.dS djzd au dd£) acoood.rc a sa o d O doooriodjoOSa^dozJdo
doods? qdj, sraado, >;U odrWd Goo zoedods zodo^O dJOci'dzwjaz • cadodoa
Sood ^dd vQnti ezoo fcedod dodo --.'coJooa.d dczg Hanrfuo drod
sradodo. ‘Soda sodCo EteS^ritf Soodd. edjoerij ^osdoio arad^ Szcra
*
drt
ddort aon^zod1 wocrari eddo^ zJSjdo. ds desJriV zlndrt deddg
SOzj^ daodoadjOjood a iSs^ddo, xodood ‘GsSzp sJoiozi’ ^eddo^do
dGdd^JDOOVO^e^dO '~^'e ^ZiOj
*
OdZ^d Esiip ade^S wIDdo^dS,, tsddoo
^ddo zadori otosjde oe3oi> j>gra dJOOTU urfoi.docJdSj eodrasrond. e
dzdodg
oda.oondi^dojoz, eod ddOZOO OJOOSSo^ Cdood TZOOO
*
djod
B2don,rit'ZZoaddjo --adoJoz dodo zizSo ?°
e-jto3OJjCjari CBddo dodoB dodou
zoooro_dzdde &oz> odd. ^dod
Soodjdo dodo adds, aoos,.
Jod^ri^wo^ zuoodsan troerfo zizdd
doodecdooo 'sddgto.
socdooa,rtvsJjoA adzdztoofid. w du,
c5zdd dood zd^ziaso wrt^ zrazj ua
riozcde _ rtjsetseo
di ^eosodaSoO ESdz^rW zori add dd zsdofcdori aoao. eo crasda
ridoodzS o/oj EdqJ Sooiooa^nvd^
zao‘V dddod dzotJ dzo“V aood ^50.
ro
dzosd wdadcortdoo «. drcdo&ra asEe^od at^aJodOj ssoOciogdoOdo.
StVeJedzd. eaomA cioozjde !Edz?d do^dOcd edo Soritf dtfrran edo
zort^ dOzdSdeiSodd
dolndSd^e Gd^ridja dJidoata aJogdKoddo, esd)
aood Sodo a>odo aodarifrt
nearf eduozMdzSo. dajjodsori rW
atao.za
^dijOd d$?va!o n®ezi dzS ?
cJ np djoodo. ednp oioSo,•< ?ideod
Zoodridoojoto.dod doodeo w«Dds
rtoocdartoo ^zSjZ bo. <^woo
zizsad&jd.
SowoddUj doo%, •aodarWo doodd aodo^.du; odd
rWoz> e&S, TaaojrWoo ezEea. euao. wddoa wdo Sorte i^drran
edjatri S. riowoQJjdo ^noddjo <^_dd aezjs zoou .doodoo^joodo «£>cn
doc^oraF aojood,ra edjicri6 ^CTaJcrio dooddzEzdzBDWodo.
ajOiogjj. EdqJrWo crasjcdoaa ddo.
rM
cjdo ©OjOJO
3oO a 53 d a
dodo
V03D d
dddJaddg wydare^d wapad osaAaa saoBajdatfnan
eroolaaAad daaeAAdda, aadadeS dsa, dedAdaa ddaQ2j;2,d©
'aUjd, dts.
tsdd, odd tadCrt ^oda tsd^odeaa jpetfddad
daaeAAVa dadaSaeddo4 zoaQ
a,d
*
: d daa/AAVa, ewarddaaeA,
eroA^oa, dddaodud daaeAAVa ^sa^O.
odd ’^0 daaaeasadfa
Adg dddJidd ‘tsda^d BdQ’Ad tsd ddradaAda acaAaa daSAdaa
rteDd dowada <ada. daodA idQd ?
sdaadde dUdaaadcd EdtfAdd.
ededaa saadAt^d <^e. adaa,e
AaaDSa^da daas,. taa^/Sa
*
adjQSca
tf.ad ddeadif daed - daed. ddcd
dJaBt^daa ‘dodaCAda daaAodaad
ddrt’ eda djadaSdjOdagdO aoda
des? dadddad od^cdada^ AaadAd
SodaAdrt ^dOj doiraosj daadco
edsad SaaU,3a. e erom,cad§ Sde£>3
EdtpAda aoaecdaartad o S ro A de
rqO I
Kjaona)deddfi dededaa daaderaAd
Sod&Ada Sada^daAd^daa egrt ssA
apadddod ae^addroio Edtfrtda S^1.
aadrS ba iS^ SoSMaO daanatj orio
^d. ZojnaA tsdda^ dd^O ddaQd
Oda^da ddaAdoe eo d^ciadda4
eaaad<A>aadL5
ddQj»dOdaa
sd
SaaL^osaAd.
esd.cia
daawda asasaddra » S.ea
u
a u
Sod
sjacaaiaadAda • dado deod
n
Saadd r<d) saJtaadS SdqSrt^AosS
enaoJaaao coda ddafc Edd Scdaod,
reatpaao' atedasa,c.
ddj da daed
id, Ada, ac^dodari z&Scjadd daed
ad^ daaradda
*
da, 3aae0?;d S3 <^da.
acda Sd ^^sartdeSouaddje ssa^
aad,t) Oddi.
a’eda^dt).
eadtavo?,
ag ddridgaa AapadsS
ux/adoa aa^o
w»ei5aazajda
SjdaasOSad, Saadadjdo. edaaeri^d
dsodg iadeda ddcdaa^d doddaa,
adafc dOe^jfiaad,decad trao uotid.
na^daS edaaert^ a> sJS, AOotoriod
ddrt add fiedddau, xapaodeadda.
dedddaLSj
AaspaC^jd
daadda
dcac4
erod.da
edaaart,6 J,Ada. adddS.o
J
daad, dadaQA«g fad. odd wddd
Ood daededdaada daaeAATrt ^dad
dad.Adaa asdd S^AUSaS.gaanaA
>^od acadadaaada Bd^Add^ aaddd,
3dvl <c^de ddad ddd,’ doda dadao
ddrt daadoa (djaOAaa
dedd dou3a,Saaet3 KdS, Uda6dd.o
Edd fcrtadod dJada^dSdeja^Afi,
en?3,da udaieA. daadSadod daada
s^Edcaa^A^) a ape? caa Ad A d sa d tr
SaadEiS Tcap adadsadS dda^Adwa^
adadaddaaj sadaijiTdud ? eotj
BdcpiAdwa^ ^esa^Aea dSa.cti? aj?
trail rtjaeajser nswcS,
ssto rijaeaasu4,
d,nf w^S’’ ^>eddas'
at, StKi^dAd,
sadaaad - ;©ooo3
aia
Rational Medicine
— A Viewpoint
By Chkar Mittal
To be presented to a group of Medicos in Delhi
This paper is the viewpoint of an individual. This viewpoint has
not emerged out of a direct collective effort.
So it carries the
imprint of all his belief & superstitions,myths and knowledge,
prejudices and convictions
The rationality of modern medicine uptill now has been an external
challenge - by proponents Homepathy, Auyurvdy and Naturopathy.
On the contraty the men of modem medicine have rejected these
systems with vehemence- being embodiment of irrational evil based
on superfctiticns and danger, their own system being the ecstatic
achievement of scientific questions. The fierce battle have led to
a truce also in favour of latter, on which we will not comment right
now.
What is more surprising is the questions being raised by men of
modem medicine themselves/of detailed discussion- grouping in
the dark. Yet this is the prime issue in front of ys
and we must
try to understand it.
The specific feature of this kind of group is that they have
a deep conviction in the inner rational core of Modem medicine,
in its firm ’Scientific Foundation
.
*
It is thought that irra
tionalities are, merely periperal, an epiphenomenon.
As one
stasts probing deeper, the distinction gets Hurred.
Che realizes
that it is not merely a question of few tonics,, cough sypsand
banned medcines being used in underdeveloped countries, few
Rhaxmaceutriggls: behaving badly in third world countries., A few
or more than few ignorant doctors who need to be enlightened
but roots of these are going very much within the rational case.
Can we separate the grain from the chaff?
In todays world there is accumulation and concentration of wealth.
There is enormous accumulation and concentration of knowledge.
At one level this knowledge has arisen out of a deep commitment
to truth
empathy with ailing & suffering humanity. At another
level knowledge (production and use of it) seems to be in service
of wealth. Vast masses of humanity get no benefit from this pyramid
of wealth and knowledge. Protest of persons like me and many others
are a cry against this state of affair a need for reaffirmation and
commitment to health and humanity,
^he pretest, the cry needs
self identification.
This cry needs a voice, a manifesto. But
common point, deposture point has to be explicity community Health
community medicine
and not an Abstract commitment and defence of
rational cere of modem medicine.
This paper is not a manifesto
in itself but mesely declaration of the intent to write one.
Against knowledge in service of
Pharmaceutical Medical Men )
filthy rich
) Nexus
For knowledge in service
of
Truth
Broad Masses of humanity.
Way to Rational Medicine
/ may be very few in number,half heasted,feeling shy
of ...
CO/WJVJU/\|/TY
■ There is a Matter-Slave relationship Slave thinks he is a slave
because the other person is a master.
The Master thinks the other
person is a Slave so he is a master.
Doctor
irrational
medicine
Pharmaecutical
Patient
~ Similarly in the triangle of irrational prescription (Tonics etc)
Doctor writes because he thinks patient demands it patient, takes them
because Doctor prescribed it Pharmaceutical manufactures because
Doctor writes it. who is at fault.
The fact -is that medical community does not feels itself collectively
responsible.
It has surrendered its collective responsibility
it lies prostrate
.
Let us take a dip into the Holy Ganges
Limits of Modern Medicine
a)
(
Rational Prescription J
Patient C/o
Constipationa
1. Laxative
•
Hyp eracidity
>
2. Zintacid ■
Dyspepsia
3. Digestan-t
Cold
4. Vi tamin
Cough with expectoration
5. Antihi s
Bronchosoasm
6.' Zmi;tihi'OtiC
Weakness
7. Bronchodilator
Lack of appetite
Can we avoid this long list.
irrational. ■
Which of them is rational and which
z
-Now if a man of Ayurveda say that root problem is in Digestive
tract and you correct it by let us say
everything else
will -go, shall- we reject it outright without giving it a f air trial
-Or if a man of Homeopathy .says that Hot uays and Cold nights have ,■
caused it and give one degejof Dukamara -200 and everything will be7 set
set right shall we ridicule it?
b)
Conceptual Foundations 4- Terms like Asthma and Diabetes
are not disease entities per se but mersely umbrella terms to
r denote identical manifestations of
i)
entirely different disease. Modren Scientific rational
. medicine groups them together because of its ignorance and
because of common symptomatic treatment available to it,
ii)
Do we know the eliology of rheumatoid arthitis but we
palliate it symptomatically
(3)
Do we really know the cause of Tuberculosis?
Yet we cure it.
So we also work empirically
then why reject the empiricism of other systems why not test
iii)
remember thalidomide disaster
remember Metronidazole has been found to be carcinogeric
in rats.
c)
Ethical basis of~ Rational basis
So we are also ignorant, we the men of Modem medicine
who have achived princples of achievements,
- Let us be humble when we speak for modem medicine
- Let us also try other systems concepts 'and practices in
our quest for truth
- Irrational practices feed and grow on the ground of
ignorance. They cannot be challenged on the grounds of
Scientif icity
or
rationality but only
Ethically
We must evolve collective ethics.
d)
Science of NutritionEmphasis is oni
Vitamin A is available in
Vitamin B Complex :
....... foods
Source
Vitamin C
:
II
Vitamin D
»
II
n
Iron
it
Calcium
Proteins
"
it
It breeds and serves consumesism of a particular class . No
concrete studies of diets of particular populations are done
to find their deficiencies.
Traditional Indian Diet (before Green
Revolution)
Complete
) Was
in 2d.l respect )
Cereal + Pulse + Butter Milk + Green Vege—
tables
available to poorest of poor
Modem diet of common man-
Pulses +Butter Milk have disappeared
We must reaffirm the custom of Donation of Butter Milk.
(4)
Limits of Modem Medicine
Cost:-.
In a limited sense the Modem Medicine can also be called
American Medicine.
Their emthod is perhaps the prototype
of scientific method or rational method.
If we see the
cost factor, this method may not be applied even to 100%
of our population.
So those concerned with community
health have to evolve a different system or a different
scientific method/rationalitywithin this system.
Iiaboratorv Investigations:
Some people claim that foundations of scientific/rational
medicine depend on a correct laboratory diagnosis.
It is
a)
It is my view point that these investigations have
co-relative significance only and no diagnostic significance
-
Sputum cluture in chronic bronchitis
-■ Asthma
arthritis :
Rheumatoid
-
Amoebic czdJJLtis - carriers
-
Diabetes
b)
Cost factor involved - Medical Industry it makes the
facility still more out of the reach of common man.
c)
Many important laboratory investigations are not done
at all
eg.
A
FS/BC therapy must always be corrobated with
Hb levels
Tuberculosis
-
Sputum AF.B/ examination culture
is more important than Xray
and culture sensitivity
Vital Statistics -
but very few T.B. Centres
in periphery are doing.
Mortality statistics indicate Higher life
expectancy
-Has morbidity deceased or increased in previous
50 or Hundred years?
- What is the indicator of improved of health of
population?
Millions of people beliezethat health of people
wasboitar 50 yrs ago.
Is.it Ian IRRATIONAL MYTH
mesely a nostalgia with past.
Shall we probe into it?
Doctors VS R.M.P.s- Some proponents of Modem Medicine (rational
version) shout at the top of their voice that
R.M.P.s should be banned and problem of irrationa
lity. will be solved.
A)
1)Large No. of them have been serving the community
since much earlier times than their Modem counter
parts.
2) In some cases they are close and more intimate
with the community.
(5)
3) Most important, they are the only medical help
available at the doorstep, still, today at the
verge of 21st century, to 80% of the people of this
country.
,
B)
a)
Some people also feel that all other systmes of
medicine irrational and must be banned.
What is ourstand?
Immunization:
Community)
Health
'
the real task is their constant training/
There is no dispute about effectivity of
immunization. But when we talk of community
health we are concerned about a different kind
of effectivity we talk of in terms of National
eradication or control
-what is role of immunization in the total concept
of primary health care
In my view the immunization crusade of WHO and Govt, of India
(complete immunization up 2000) has laregely/apto 2000.
Change
of emphasise has led to empty sloganism.
We must not forget that
these vaccines are not the one which are necessary but are the
ones which are available.
b)
Do we agree with the Governments Model of providing Primary
helath care in-the village?
Is it rational?
It is supposed to
depend on village level worker and not on village level trained
Doctor.
c)
We produce 20,OOOdocotors every year 10,000 of them of Modem
Medicine. Their syllabi are more appropriate to pass ECFMG and GMC.
In ICMR study group has recommended incorporation of alternative
systems of medicine at undergraduate level I incorporate vetenaxy
medicine also as supporters of Rational Medicine, What is our
stand on Medical Education in India ???
Thought the history of Modem meidicine's conceptual development
is 150 yrs old, its present form of practice is a post 2nd World
War phenomenon- about 45-50 yrs only.
Its pace of development has
been very very fast. It goes to the credit of medical men of our
coutnry that they have not lagged behind in this race.
But perhaps
the time has come to halt, to rest, to assess to review to absorb
especially our traditional systems of Healing as well as others like
Homeopathy and acupuncture to make our own conceptual contributions
to feild of Medicine in areas like Tuberculosis or may be Cancer
and not be dependedt on Zmierican for shwoing us the light, to have
our own methodology of community health and medicine and not an
imported version like green revolution.
/.replaced the goal of Primary Health care to everybody
RATIONALITY OF
MULTIVITAMIN
PREPAR.aTI DHL
Part 1 - The Myths of Multivitamins of children
Dr. Anita Srivastava
The word "VITAMIN" refers to organic compounds which are required
in minute amounts to catalyse cellular metabolism essential for
maintenance or growth of the organism
They must be supplied
wholly, or in part exogenously.
Recommendations for daily intake of Vitamins
1
Ascorbic
Acid
D
Bij
nig.
IU
mg.
8
35
4 00
0.4
9
45
4 00
0.8
0.8
11
40
4 00
0.8
1.2
1.4
16
45
4 00
1.0
10 -12 3300
1.3
1.6
18
50
400
1.2
13 -15 3300
1.5
1.7
18
60
400
1.4
Niacin
Age
A
Thiamine
Riboflawin
Year
IU
nig.
rng.
rng „
1 year 1300
0.5
0.6
2-3 1300
0.7
0.8
4-5 1650
0.8
- 9 2300
6
(Recommended Dietary Allowances, Revised 1.930, National Research
Council, National academy of Sciences).
Properties and Food Sources of the Vitamins
Vitamin A
Retinol
;
Provitamin A
:
Plant pigments, Alpha, Beta
and Gama carotenes
and cryptoxanthines.
Characteristics : Fat soluble
Effects of deficiency :
Nyctalopia, Xerophthlmia-,
Keratomalacia,
leading to blindness faulty epiphyseal bone formation defective
tooth enamel, Keratinisation of mucous membranes and skill retarded
growth.
Effects of Excess ;
Dietary excess of Vitamin A-unlikely. Excessive
carotene intake may produce carotenemia with xanthosis cutis.
There
is- individual variation in sensitivity to high intakes of Vit. A
concentrates.
50,000 I.U.
Anorexia,
taken daily for prolonged period may be toxic and cause
slow growth, drying and crackling of skin, enlargement of
liver and spleen,
sv/elling and pain of long bones,
bone fragility,
increased intracranial pressure.
Recommended allowances e
Upto 1 ycar-1500 IU/day;
1-2 years -
.2000 IU/day, increasing to 1500 IU with age,over 12 years-5000
IU/day.
These amounts assume that 2/3 comes from the provitamins,
•which are less efficiently utilised than the vitamin.
If only
Vitamin A is taken,then 900 to 3000 IU/day would suffice.
contd.... 2
/2/
Liver,
Sources :
fish liver oils,
whole ini Ik, milk fat products,
\
egg yolk, carotenoids from plants - green vegetables, yellow fruits
and vegetables.
VJTAMIN B COMPLEX
COBALAMIN : Group of complex coordination compounds of Cobalt
Vitamin B12-
Characteristics :
Slightly soluble in water
destroyed by light.
Effects of deficiency : Juvenile pernecious anemia.
Effects of excess :
Unknown
Recommended allowances : Infants s
1-2 micrograms.
Children (1-18 years)
:
Sources
b6
:
muscle and organ meats,
3 active forms -pyridoxin,
fish,
2-5 micrograms.
eggs, milk, cheese.
pyridoxal and
pyridoxamin.
Characteristics : Water solublem,destroyed by ultraviolet light
and
by heat.
Effects of deficiency :
Infants - irritability, convulsions,
hypochromic anemia,
perepherel neuritis
Effects of excess s
Unknown.
Recommended allowances ;
Infants - 0.2 to 0.4 mg.
Children - 1 to 10 years ;
If abnormal
Ln patients receiving INH.
0.5 to 1.2 mg,
10 - 18 ye rs:
1.4 to 1.3
Bg metabolic state exists 5-10 mg.
Sources :
Meat, liver, kidney, whole grains, peanuts, soyabeans.
F0LACIN
Characteristics : Slightly soluble in water, labile to heat and ligh
Effects of deficiency :
Megaloblastic anemia particularly in
infancy and pregnancy.
Effects of excess
Unknown
Recommended allowances :
Sources :
.NIACIN :
20-50
microgram/day
Liver, green vegetables,
Nicotinamide,
Characteristics :
nuts,
cereals,
cheese.
nicotinic acid.
Water soluble,
heat and light stable
Effects of deficiency : Pellagra
Effects of excess ;
Nicotinic acid (not the amide)
is vasodilator,
reactions include skin flushing and itching, circulatory disturbance
increased peristalsis.
Recommended allowances : 6.6 mg/1000 calories
Sources : Meat,
fish, paultry,
cereals, green vegetables,
liver, whole grain and enriched
peanuts, protein food in general,
from
conversion of tryptophone (60 mg from 1 mg of niacin)
contd.......... 3
:
■ RII30FL.AVIN
Vitamin B2
Sparingly soluble in water,
Characteristics :
sensitive to light,
stable to heat.
Effects oE deficiency :
Ariboflavinosis; early symptoms are
photophobia, blurred vision,
burning and itching of eyes, corne-jl
vascularisation poor growth.
Ono of the most common dietary
inadequacies
often accompanying other 13 vitamin deficiencies.
Effects of excess : Not harmful
Recommended allowances :
Milk, cheese,
Sources ;
0.025 mg/gm of dietary protein
liver and other organs, meats, eggs,
fish,
green leafy vegetables, whole or enriched grains.
THIAMIN
: Vitamin Bl
Characteristics : Water soluble,
Effects of deficiency :
Effects of excess :
Beriberi
lione from oral
Recommended a 1lowances :
wheat germ,
legumes,
pork, milk,
Character iti c» z
whole grain or enriched
pests.,
(Please refer page No.
BIOTIN :
intake
0.4 mg/1000 Kcal
Sources : Liver, meats, esp.
cereal,
hoat labile
14)
crystal 1 i :;< -d From yeast
'liter :;<> I nbl. >,
Effects of deficiency : Dermatitis, Seborrhoou
Effects of excess : Not known
Recommended allowance :
Source :
Yeast,
VITAMIN C
.; Ascorbic Acid
animal products,
Characteristics :
Water soluble,
Effects of deficiency :
Effects of excess :
easily oxidised
Scurvy and poor wound healing
No£ harmful
Recommended allowances :
Sources :
synthesised in intestines.
35 to 60 mg depending on age and sex
Citrus fruits, Tomatoes, berries, cabbage, green vege
tables, cooking has deletariaes effects.
VITAMIN. D :
Group of sterols having similar physiological activity.
D2
- Calciferol is activated ergosterol
D3
- is activated 7 dehydrocholesterol
Characteristics :
Fat soluble,
stable to heat and oxidatioh
■
con td.....4
/4/
Effects of deficiency :
Rickets,
infantile tetany poor growth,
osteomalacia.
Effects of excess : Vide variation in tolerance.
In general
20,000 to 50,000 lU/day is toxic when continued for weeks (prolonged
administration of 1800 lU/day may oc toxic) .
Manifestations are nausea,
diarrhoea, weight loss,
renal tubules, blood vessels, bronchi,
heart,
Recommended' allowances ;
including
stomach.
4 00 lU/day
Sources : Vitamin D fortified
oils,
polyuria,
ccalcjf ication of soft tissues,
nocturia, eventually
fish liver
milk and margarine,
exposure to sun light or other ultraviolet sources.
VITAMIN
Group of related chemical compounds tocopherols - having
E ;
similar biological activity.
Characteristics ; Fat soluble, heat stable in absence of oxygen.
Effects of deficiency ; Anti-oxidant important to cell membrane-
I
integrity, endoplasmic reticulum and mitochondrial oxidative
function.
May be involved in red blood cell
hemolysis in premat tie
infants.
Effects of excc:;s : Unknown
Recommended a 1lowances : Requirements related to polyunsaturated .
fats intake.
Infants - 5 IU, Children - 1-6 years :
6-10 years :
15 IU)
Sources :
nuts,
10-14 years s
10 IU
14-18 years :
20 IU,
25 IU.
Germ oils of various seeds, green leafy vegetables,
legumes.
VITAMIN K
• Group of compounds, napthoguinones with similar
biological activity
(
is phylioquinone
Characteristics : Natural compounds are fat soluble but several watvi
soluble products have been developed (menadione)
Effects of deficiency
stable tq heat.
Hemorrhagic manifestations are result of
faulty intestinal synthesis of Vitamin K (new born,
of antibiotics)
synthesise
prolonged use
faulty intestinal absorption or inability co
prothrombin
(hepatic damage)
.
Dicumarol and salicy
lates act as .Vitamin K antimetabolites .
Effects of excess : Not established medicinally, may produce
hyperbilirubenemia in prematures.
Recommended allowances
Not a dietary problem.
1
to 2 mg/day
appear to be adequate.
Sources : Green leafy,vegetables, pork liver, widely distributed.
contd.....5
/$/
'
!
I
I
The U.S. Food and Drug Administration (FDA) has estimated that
40% of adults in USA take Vitamin supplomtns on a daily basis.
(FDA Drug
vitamins
Buu.
13:27,
1983) most of
these p-ople do not need
(but multivitamin preparations
can bo useful for patients
on highly restricted diets or those with increased nutritional
requirements).
Healthy adults on v.ried diets that maintain their
body weight ordinarily do not need vitamin supplementations.
PREGNANCY :
Requirements for vitamins, particularly folic acid,
are increased during pregnancy and lactation.
Supplemental vitamins
with iron are often prescribed to ensure that these requirements
C,;
i
are met, but except for 0.4 mg of folic acid daily throughout ge.-tation (Medical letter 14:50, 1972)|there is no well established need
to take supplements of other vitamins during pregnancy and vitamin
overdosage may be harmful to the fetus.
A diet that includes green
or yellow vegetables contain sufficient amounts of Vitamin A and
[ "
more than 25,000 IU per day can cause congenital malformation.
Enough Vitamin D to meet requirements can be obtained from seasonal
I
exposure to sunshine or from four cups daily of vitamin D fortified whole or skin milk.
Overdose
of vitamin D in pregnancy can
'
cause
aortic stenosis, hypoparathyroidsu and other caugenital
malformations in the new born infant.
vitamin C available in one
One hundred
ments of pregnant women, more than 1 gram per day
the
mg daily of
cup of orange juice, meets the require
may condition
uetus to a high Vitamin C environment and cause scurvy in the
new born (Medical letter 20:65,
1978).
It has been suggested that vitamin mineral supplements may help
prevent several tube
Lancet,
1:1027
defects in the new born (RW Smitheils it al
1983).
INFANCY & CHILDHOOD :
All
new born infants should receive Vitamin
K once (0.5 to 1 mg or 1-2 mg orally)
to prevent'.hemoranagic dise-iso
of the new born.
Premature infants weighing less than 1.5 kg should
receive vitamin E
*
(500/
e^/kg daily)
to prevent hemolytic anemia.
Exclusively
breast fed infants may need supplemental vitamin D
(400 lU/day)
if they have limited exposure to sunlight.
i
i ■
.
Breast fed
infants whose mothers have been strict vegetarians for several
years may need vitamin 3^2
66:1015,
(Committee on Nutritian, Pediotrics
1980).
Empirical experience suggests that fully breast fed infants nursed
by reasonably nourished mothers will receive adequate amounts of
nutrients with the possible exception of vitamin D for the first 4
months or sol
In the northern U.S. and Canada there is evidence
to suggest that breast milk may not contain adequate amount of
Vit. D, a supplement approximating the recommended intake level is
contd....6
often suggested.
Most,
if not all,
infant formulae contain au.'.ed
Supplementation should not be necessary
Vitamin D.
Tor the
formulated infant except in the cas • of very low birth weight
or premature infant who consumes much less formula and hence less
Vitamin D and who may have a higher requirement for the vitamin
< UtV (A
Vs)
(2.)
Tabled
.
Computed Nutrient Density
Criteria by Level of Coverage
for infants
KTIim^Tr,v,m
NUTRIENT
(.units;
50%
Protein g/1000
kcal
16
1
18
20
25
15
17
19
29
Thiamin mg/1000
kcal
0.3
0. 34
0. 37
0.4
0. 3
0. 34
0.37
0.4
Riboflavin mg/
1000 kcal
0. 38 0.42
0.45
0.51
0. 38 0.42
0.45
0.5
Niacin
Niacin equiv./
1000/kcal
5.5
6.1
6.6
7.1
5.5
6.1
6.6
7.1
Folacin /-(.g/1000
kca 1
42
48
55
64
47
54
62
73
Vitamin Bi2?-(g/
kcal
0.46 0.55
l
1
)
0.67
0.85
0. 33 0.4
0.48
0.61
Vitamin C mg/
1000/kcal
0
36
44
56
21
26
31
40
Vitamin A
600
Retinol equiv./
1000 kcal
750
900
1150
450
525
650
Vitamin DM g/1000 15
kcal
19
22
28..
11
13
16
20
Vitamin E mg/1000 4.6
kcal
5.5
6.7
8.5
3.3
4
4.8
6.1
Calcium mg/1000
kca 1
650
780
990
38
46 0
560
710
’ADEQUATE TO COVER ALL
■
0UT
5Q%
25%
10%
2.5%
ADEQUATE TO'COVER ALL
3UT
25%
1Q/o
2.-5%
3 - 5.9 months
0-2,,9 months
540
Iron mg/lOOOkcal
0.6
0.72
0.87
1.1
Magnesium mg/
1000 kcal
49
57
65
76
Zinc mg/lOOOkcal
3
3.6
4.4
Vitamin Bgj<g/gm
protein
11.5 12.7
13.8
■
800
5.4
6.5
7.9
10
47
54
62
73
5.6
3.3
4
4.8
6.1
15
11.5
12.7
13.8
15
.
6 - 8 months
9 - 11..9 months
Protein gm/1000
kcal
16
17
19
22
15
17
19
21
Thiamin mg/1000
kcal
0.3
0.34
0.37
0.4
0.3
0.34
0.37
0.4
Riboflavin mg/
lOOOAcal
0. 38 0.42
0.45
0.5
0. 38 0.42
0.45
0.5
Niacin Niacin
equiv./lOOOkcal
5.5
6.6
7.1
5.5
6.6
7.1
6.1
6.1
contd,,.. .7
•
/V
Table - 1 Coned.
ADEQUATE TO COVER ALL
BUT
5 0%
25%
10%
2.5%
NUTRIENT
(Units)
A DEC UATE TO COVER
3UT
10%
50%
25%
6 8 months
Folacin ».(g/1000
kcal
48
Vitamin BipA'-o/
1000 kcal
0. 29' 0.35
Vitamin C mg/
1000 kcal
19
9 - 11.9 months
63
74
47
0.4 2
0.53
23
27
Vitamin A Retinol 400
equiv./lOOO kcal
450
Vitamin D/‘ig/
1000 kcal
9.6
Vitamin E mg/
1000 kcal
Calcium rng/1000
kcal
62
72
0. 24 0. 29
0. 35
0. 1 ■.
35
16
19
23
29
550
700
325
400
450
600
12
14
18
3.1
9.8
12
15
2.9
3.5
4.2
5.3
2.4
2.9
3.5
4.a
385
465
560
710
325
390
470
600
55
54
Iron mg/1000 kcal 6.8
8.1
9.8
12
5.7
6.9 '
8.3
11
Magnesium mg/
1000 kcal .
48
55
63
74
41
47
54
63
Zinc mg/lOOOkcal
2.9
3.5
4.2
5.3
2.4
2.9
3.5
4.5
Vitamin
g/
gm protein
11,5 12.7
13.8
15
11.5 12.7
13.8
.15
Derived from -Recommended Nutrient Intakes for Canadians"
Recent evidence shows that at least the first four months of life,
full breast feeding by- an adequately nourished mother gives assure -.c
of adequate nutrient intake, for almost all infants.
This
'epidemic-
logical evidence may suggest that there is need to reconsider someof the current estimates of infant requirement.
(2)
If a formula or a feeding mixture falls seriously short of the
recommended criteria consideration could be given to prophylactic
supplementation.
The level of supplement indicated would be less
than the recommended nutrient intake,
not devoid of the nutrient(s).
since the formula or mix is
a
In such/situation a practical
-e to offer a supplement every other day or perhup
approach might
'
twice a week,
using the
ming that the
'usual dose' provided’a level of nutrient approximate
'usual dose
suggested on the label (assu
the recommended nutrient intake). • Judgement on whether or not s .c.
supplementation is indicated could be based on the levels of cov.r
recommended in the above Table,
as estimates of the likelihood ths-.
the formula or mix is adequate for the infant at hand a random m-.d --
of the population of similar infants.
If the risk is relatively la-’
the practitioner may deem that the chances of any benefit of supple
mentation is too low to justify either the cost or effort of the
mother.
(2)
•
contd....;’
/Q/
Table - 2.
Approximate composition of Hainan milk and Cow's
Vitamins
(litre)
(i)
milk
Human milk
Cqw's mirk
Vitamin A (IU)
1898
1025,
Thiamine (/Ag)
160
440
Riboflavin (y(g)
36 0
1750
Niacin G-tg)
14 70
94 0
100
64 0
Pyridoxin
(y-.g)
Pantothenate
(mg)
2
3
Folacin (Mg)
52
55
Vitamin C (mg)
43
11
Vitamin D (IU)
22
14
2
0.4
60
Vitamin E (mg)
Vitamin K
(Aig)
15
•
Some vitamin can interfere with the effect of commonly presort -ed
drugs and some drugs may increase the dosage requirements ter ,i
vitamin.
Information in this area is still limited
however,
a;-..-
the absence of a listing in the table below does not necessarily
me'n that no interaction will occur.
Table - 3
VITAMIN - DRUG
Vitamin
Folic acid
Niacin
Pyridoxine
INTERACTION..?
Drug
(3)
Interaction
Phenytoin
Decreased phenytoin efJ.A
decreased dietary foist,
absorption
Sulfasalazine
Decreased dietary fol.-.
absorption
Triamterene
Decreased utilization
dietary folate
•
Zinc
Decreased zinc availed.' .
Isoniazid
Niacin requirement may ,
increased
Barbiturates
Decreased barbiturate .
Contraceptives,
oral
May increase pyridoxin.'
requirement
Hydralazine
May increase pyridoxin.
requirement
Isoniazid
May increase pyridoxi'..
requirement
Levodopa
Decrease levodopa off..--:
(but not with carbidop -
Penicillamine
May increase pyridsxir..
requirement
Phenytoin
Decrease phenytoin
contd.... 9
/s/
Table - 3 contd.
Vitamin
' Drug
Vitamin A
Anticoagulants,
oral
Vitamin C
Anticoagulants,
' oral
Occasional decreased anti
coagulant effect
Contraceptives,
oral
Increased serum concentra
tion and possibly adverse
effects' of estrogen with
■ 1 gm/day of vitamin C
-■
Interaction
Increased anticoagulant
effect with large doses
vitamin A
? ■ ■-Estrogens'
..1”
•
Increased serum concentra
tion and possibly adverse
effects of estrogens with
1 gram/day of vitamin C
■
Decreased phenothiazine
effect after vitamin C
deficiency corrected
Phenothiazines
Decreased activity of
vitamin D
, . Phenytoin
Vitamin D
- -
Phenobarbital
Decreased activity of
vitamin D
Vitamin E
Oral anticoagu
lants
Increased anticoagulant
effect
Vitamin K
Oral anticoagula
nts
Decreased anticoagulant
effect
Vitamins
Cholestyramine
Decreased vitamin absorpt b-
Colestipol
Decreased vitamin absorptiu
Neomycin
Decreased vitamin absorpti?
Fat soluble
Vitamin dependency states require higher than usual dosage of vitamins.
Table - .4
Disease
Vitamin
daily
dose
• Untreated state
Hyperkeratosis folli- 25,000 I
aulasis
A
Darier
Bl
Leigh-pyruvic-lactic Ataxia,
.acidosis
retardation
6 00 m
Thiamine responsive
anemia
Megaloblastic andmia
Maple syrup urine
disease
Hypotonic,
Riboflavin
Pyruvate Kinase
deficiency
Hemolysis
10 :
Niacin
Hartnup
Ataxia, Eczema
200 m
•Cystathiaminuria
No symptoms
200 i:
Homoc ystinuria
Retardation
200 m
Bg
Hypochromic Micro
cytic ar ...mia
B6
anemia
seizures
20
10 m
10
contd... . 10
/10/
Table - 4 cont'.;.
Vitamin
Disease
Bg
Seizures
Seizures
■tauthuremic aciduria
Gyrate atrophy of
choroid
Folic acid
b12
Biotin
Daily,
dose
Untreated state '
25 mg
10 mg
retardation
. Blindness
100 .mg
Oxaluria
Oxalate crystals
Formiminotransferece
deficiency
Retardation
5- inc:
Folate reductase
deficiency
Megaloblastic
anemia
5 mg
Homoc ystinuria
Retardation
10 mg
Methylmalonic
acidemia
Retardation
1 mg
Propionic acidemia
Retardation
. 10 mg
Beta Methylcrotonyl”
glycinuria
~
CGlUcl
100 mg
10 mg
C
Chediak-Higashi
Infections
D
Dependency
.Rickets
Familial hypophosphateuria
Rickets
5 0 mg
4 000 .; '
100000 I
Vitamins in Malnutrition
With an adequate therapeutic regime no specific dietary supplement
are usually needed although they may be employed if a particular
vitamin deficiency is known to be
common locally.
doses of vitamin A should be given I.M.
of vitamin A deficiency are present
Therapeutic
initially when occular sig
because the
gress very rapidly to irreparable damage.
lesions can pro
Folic acid administra
tion might be required in cases with megaloblastic anemia.
According to certain surveys,
(4)
the prevalance rate of B-complex
‘
deficiency is 5% in. pre school children and 17.8% in pregnant womc.
(assessed by the presence of angular stomatitis and glossitis)
(9)
The prevalance of vitamin A deficiency is high in the eastern and
southern parts of India and the main suffei er is the underprivilu.:
class.
The age group most affected in our country is between
1-5 years and as many as 5 - 10 %
of the toddlers have been, st
to be suffering from clinical deficiency.
(10)
Vitamin A Prophylaxis
Prophylaxis should be directed at the 'at risk'
situation.
Here
again the massive dose is the earliest and most efficient
way of
action,
£very marginally fed child
suffering from diarrhoea,
apetite reducing pyrexia, measles etc., can be protected at least
from xerophthelmia, by one'Single therapeutic oral or I.M. dose.
contd.
11
/Il/
Safeguarding the newborn infant might be achieved by giving ’aim
50,000 I.U.
orally or 3,00, 000 I.U.
to the lactating mother aft..r
delivery.(4)
.has been practiced in India.
Mass prophylaxis of xerophthalmia
and Bangladesh in child population between 1 to 4 years every
The massive dose of 2,00,000 - 3,00,000 of retinol in
6 months.
oil by mouth is sufficient to maintain a satisfactory serum lev.1
for at least 3 months
W.H.O.
(it also fills up the liver stores)
(4)
recommends that in areas of the world where vitamin A
deficiency occurs 1,00,000 IU of vitamin A be
water miscible base 4 times yearly.
The same
postpartum to mothers of breast fed infants
TH CRAPY IN VITAMIN DEFICIENCY DISEASES
'
given orally in a
_cse should be given
(1).
(4)
Vitamin A deficiency - Xeropthplmia
The purpose of treatment is the rapid restoration of vitamin A
liver stores and its mobilisation into the blood and lymph streams
X2 or X3 A when changes are still
Delay for even one day at stage
reversible, may make all the difference in future vision capacity.
Serious corneal lesions in hospitalised children should bo treated
with deep I.M.
one year)
injection of 2 x 1, 00,000 IU.
(half in infants un.:. :
of the water dispersible preparation for quickest action.
The oil solution is not suitable for parenteral use as the vitamin
is liberated extremely slowly.
route is next choice ;
But the oil solution by the oral
Slight transient signs of toxicity (eg.
vomiting) can be disregarded.
(4)
Every confirmed or dubious corneal lesion of this kind should be
hour
treated within the
it is discovered by any cadre- of medical
personnel.
Improvement must be evident within 5 days, otherwise
diagnosis becomes doubtful.
The nonperforating lesions are easily
reversible.
There is no sense in local application of retinol preparations.
Thiamine deficiency - Beriberi
In seriously ill patients,
given I.V.,
I.M.
25 mg of Thiamine should be slowly
then a further injection of 25 mg should be administer
Thereafter,
20 mg should be given orqlly or I.M. once or twic.
a day until major symptoms have subsided
after which an oral d-.
of 10 mg daily for several weeks should be maintained.
contd.
/12/
In adults or older children,
a
higher initial dose of 100 mg
could he given without toxic symptoms.
However,
in seriously
ill patients, an attempt should he made to inject slowly,
the initial dose
. l.V.
part of
to get an immediate maximal effect.
In infantile beriberi, which almost occurs among breast fed
infants, mothers should also be treated daily with 50 mg thiamin
orally (or IM for the first few days)
Niacin deficiency -'Pellagra
10 to 20 mg may be given thrice daily as tablets.
is
Nicotinamide
preferable as it produces less burning sensation in the skin.
Riboflavin deficiency
Ariboflavinosis is quickly cured by administering 2-5 mg tablets
daily,
of the pure vitamin.
Folic
acid deficiency
2-5 mg/day .
.
Treatment should be continued for 3-4 weeks.
I
Satis
factory response's have been obtained with dose of 5 0/<g/day (1)
Vitamin C deficiency - Scurvy
uptg. 1 gm may be given as
In the established case,, large amounts
tablets in a single dose.
There is little to recommend the tra
ditional but cautious administration
of 50-100 mg thrice daily,
unless a loading dose has been previously given.
Vitamin D deficiency - Rickets _
(1)
The administration-of 1500/tgm of vitamin D in a single dose,
without further therapy for several months
This is followed by
more rapid healing
is advantagesuus .
and less dependence on
the parents for daily a 'ministration of the vitamins.
ling occurs Rickets is probably resistant to vitamin D.
healing is complete vitamin
(1/M.g
"
If no he.-After
D should be lowered to 10/<g daily..
= 40 IU)
MISUSE OF MULTIVITAMINS
"Probably no single class of drug has been the target of as much
quackery, misunderstanding, misrepresentation and misuse as the
vitamins"
(9).
Among the pharmaceutical preparations that are
indiscriminately prescribed are the vitamins,
particularly thos-
)Of the B - complex group^
Patients often come with vague symptoms which can be correlated .
to no known disease.
So one usually prescribes a multivitamin or
B-complex preparation because the physician may sincerely believe
that vitamins will help the patient or he may feel compelled to
prescribe something.
contd....!3
•
/13/
The trouble arises with the dose that is prescribed.
physician should realise
that
The
in such undefined situation,
the therapy is purely empirical.
The burden rests on him to
know whether he has prescribed the right amount,
less or more.
One must differentiate between vitamins taken as nutrients to
those
ward off deficiency and/taken for therapeutic purposes, in esta
blished deficiency.
Since water soluble
dered to be relatively innocuous,
high.
vitamins are consi
the amounts prescribed are very
An argument imay be put forward that since water soluble
vitamins are harmless compounds there is no need to raise a hue
and cry about the dosage prescribed.
This may be true but,
‘such practice is economically wasteful and in same - instances,
causes financial hardship1.
Vitamin therapy is often given to patients wit^golyneuropathy,
although it is clear that polyneuropathy is
/_
due to deficiency
of vitamin Bp Bi2 nor any other known vitamin. Such treatment has
only a olacebo value.
(9)
Several workers have advocated large doses of water soluble group
of vitamin to improve molecular composition and function of brain
and also for schizophrenia.
Except at extremely low vitamin con
centrations or very high concentrations
centrations are relative y constant.
the excretory mechanisms
the brain vitamin cn-
At very high conccntratio s
in kidney operate and I.V. injections
are required to produce very high vitamin levels.
Even djhen,
it
is still doubtful whether megavitamins help to improve cerebral
functions.
Only in certain conditions such as retarded children
folate transfer mechanism
are reduced.
Whether high folate
therapy can increase brain folate levels in such
affected transport mechanisms is still
children wirh
debatable.
During menin
The use of
gitis also the transport mechanisms are suppres ed.
high dose vitamin therapy in such cases remains to be established
MULTIVITAMINS AND THE DRUG INDUSTRY
In India,
126 vitamin B12 preparations are available - the cost
of a day's treatment with multivitamins in India and Bangladesh
is equivalent to 20 to 30 per cent of the average daily wage.
(5)
In India 40,000 children become blind each year because of vitami
A deficiency, which is a leading cause of blindness in pre-schu.-l
children.
bility.
It is associated with 6-12 times higher infant insta
With 42% of India's population below the age of 15 ye -is
and with children population increasing, vitamin A requirement
would necessarily increase.
Unfortunately vitamin A production
has not merely NOT INCREz\.SED BUT ACTUALLY DECREASED.
(6)
contd...14
PRODUCT FORMULATION AT '.'HOSE COST ?
Sector wise shar.. in Bulk drugs and formulations.
in four Anatomical Groups in 1978
Production
(6).
Therapeutic group percent contribution
Indian
Pvt.
Foreign
Public
1. Vitamins
Market share
79.3
18.6
2.1
Bulk production
7.2
82.3
10.5
38.4 ■.
57.8-’-B-
11.0
49.0
46.3
51.3
2.4
4.0
72. 0
24.0
2. Antibiotics
Market’ share
■
Bulk production
3.8
40. 0
.3. Analgesics
Market share
.
Bulk production
4. Anti Parasitic
Market share
67.1
31.5
1.4
Bulk production
37.0
49.0
14.0
REFERENCED ;
12th Ed.
1983
1.
Nelson Text book of Pediatrics
2.
PCNA, Vol.
3.
Medical letter 273 66-68, 1935
"
f■ V
Disease of children in the subtropics and tropics Ed. by
D.B. Jelliffe & J.P. Stanfield, 3 Ed.
4.
32 No.
2, April 1985
March 23,
p.
5.
Indian Express, Magazine
6.
R.-tional Drug Policy : Facts and Figures
AIDAN,March 1986
7.
Nutrition Atlas of India (C. Gopalan & K.V
NIN Hyderabad 1971
8.
'Brain Tonics' by Dr. B.P. Udwadia
Handout of Academic
Society'of K.G.P. Children Hospital andJ-ajodia Research
Centre,' Baroda.
9.
'Tonics : How much
an Economic Waste'
Kamala S Jayarao; MFC Bulletin, Nov. 1976.
10.
Nutritional problems in India,by P.K.Shukla, 1982.
5iori.r? •.
1986
3
Raghavan
i Ed-..
(Contd.from Page 3)
Daily requirement of Biotin in adults has a provisional valse of
100-200/<g as assigned by Committee on Dietary Allowances.
In infants
: upto 6 months
:
35/^g
:
5 0,-< g
153 years
x
65/.. g
4 - 6 ye ars
;
6 months to 1 year
In children:
7-10
"
85/-( g
: ' 120.,\ g
11 years onwards
;
100 - 200.\g
(Goodman and Gilman)
It is found in'adequate amounts in our diet.
A part of it is als
available from what is synthesised by bacteria in the intestines.
Biotin deficiency is not known in humans.
Clinical deficiency
syndrome has not manifested so far
The vitamin has no clinic.’!
application.
Use And Misuse of Oestrogen-Progestogene Forte Combination
Use as suggested in the past :
>
1.
Hormone testing for pregnancy.
2.
To bring on a delayed period.
3.
Abort!facient.
1,
The drug companies themselves agree it is not to be used.
A.
However, the idea has got so firmly ingrained in the minds of
the medical profession and general public that if the drug
continues to be available, its misuse will be hard to police.
B.
Simpler and simpler pregnancy tests are available, which do
not require much skill and do not require refrigeration for
storage.
C.
They are cost effective (Less Rs. 17/- per test).
It is generaly believed that if the woman is given this
preparation in the event of pregnancy, she will not menstruate.
There is evidence to show that 18.9% of females who did not
menstruate were not pregnant (false positives).
D.
The drug has a potential teratogenic effect on the foetus.
In the event that the woman wishes to continue pregnancy
after taking the injection, she finds herself in a dilemma of
i) either bearing a child with the possibility of birth
defect/defect which may be manifested later in life (?) at
puberty or ii) going ahead with termination of pregnancy
with all its social, medical and psychological implications
for her.
2.
In the normal menstrual cycle, menses is brought about duo
to a fall in the circulating level of the hormone progesterone.
In case of a delay in menses, in a female
who otherwise menstruates regularly, she may either a) have
not produced progesterone at all or b) the level may not
Contd ....2|
have reached the low threshold level,.
In either case there
is no place to give her a drug containing both Oestrogen and
Progesterone.
Moreover, the drug may furtherdelay the fall
in her hormone level and hence delay her menses, even
further.
In the case of a woman who does not menstruate duo to very
low endogenous production rate of Oestrogen, this short
course of exogenous Oestrogen is not sufficient to stimulate
endometrial growth and subsequent shedding.
There is on-going research to discover an oral hoi'monal
abcrtifanient
and the future holds promise of a drug
containing high doses of hormonc(s).
However, this would
have to be taken soon after intercourse as it acts by
preventing implantation of the fertilised egg within the
uterus.
The side-effects of the drug are too severe to
recommend its use as a regular contraceptive and it may be
helpful only in occasional cases.
In any case, the drug
being tested has a hormonal concentration several times
that found in the drug Oestrogen-Progesterone forte.
DRUG SITUATION IN INDIA
A Report of the workshop
held in Mysore.
DATE
:
17.09.1989
VENUE
:
Madhava Kripa
J.L.B.Road
MYSORE.
SPONSORS :
NATIONAL
MEDICOS
ORGANISATION
MYSORE UNIT.
(? 0 Q 0 0 0 0 0 0 © G 0 O 0 0 0 0 Q 0 0 0 Q O 0 0 0 O 0 0 G 0 0 0 0 Q 0 0 0 0 0 O : 0 0 O O O 0 0 0 0 0 O 0 0 0
^oeooeoooeseB cooG ooeoeooB saooeaoeesooeoB O saasoesoeooooo®
eooeo0000O00oo000Q0o000O0O00©000000O000oo0oo0O0oo©oo000O0oo
BSBooooeoBosBaseoGsoooooooBoaBoooooaBeBBoaooooooosaBBOBOBsoi;
2
Drug situation and the health care delivery system have
3.
become a scourage.
possible,
We
intend to analyse it in all its aspects,
so that our emerging young professional becomes bitter
informed, wnile doing his work "or the society,
and the nations.
That is the ultimate purpose of this workshop, as also of the one
held last year end the future workshops to come.
Dr. Shirdi Prasad Tekur started the initial "running in" and
"warm up" on what exactly is health was greeted with an unexpectedly
wide range response - sound in its quality which went far beyond -the
scope of even W.H.O definition.
Everyone is aware of the physical, mental and spiritual well
being as health.
But to achieve this students came out asking and
telling.
There is an economical health,
health,
family health;
political health,
i
community
they came out with environmental health als»;
It was obvicens that their thinking had a holistic pattern
which was heartening.
" THE R^MAKKA STORY "
A videofilm was creened.
It was felt essential to have a
glimpse of how Indian health care system actually functions at the
grass root level.
Ramakka's story is the story of a farmer's wife in
a remote village havin g a husband, working out of the house and two
'children, One five and one year.
Ramakka also works and the elder
child looks after the younger one.
The younger one starts with frequent diarrhoeal stools,
absence of Ramakka.
in
The elder child looks after the young one,
what is possible, but the loose motions do not stop,
feeds the child and tries to give medicine,
"Mantravadi" on payment.
feeds
Ramakka returns,
through a local
The diarrhoea does not stop.
It worsens.
The manthravadi has not even had a conversation with Ramakka,
but
for some mumbling.
•
Fearing that the child is becoming inactive and warse,
goes to a local general practitioner.
for it,
Ramakka
The doctor gives an injection
on payment gives away some medicine,
does not talk nor explain
anything about the disease.
The diarrhoea continues and the child worsens,
gather,
Villagers
the husband has not returned as he does not even know. Taking
the child to a government hospital is suggested.
main road,
It is far off;
the
to get a precarious bus on, which may not be coming up for
a number of hours,
is itself a few kms away.
Even then Ramakka starts
... .3.
3
The baby is dehydrated.
After several hours Ramakka reaches
For this she has already borrowed money from
the Govt. Hospital.
the head of the village.
her to see the doctor.
hospital comes in the
The attendee of the
way, scolds her for not comming up in time.
He is not ready to allow
Again o.i payment she is allowed by the attend
ant to reach the doctor.
The doctor's timings are over.
He has to be located first.
Without bothering to find out why she has come late, without' under
standing her difficulties the doctor also fires her, while he has
not even paid cursory attenction to the badly dehydrated child.
Ramakka manages to explain delays and difficulties.
doctor condescends to accept to see
the child,
Doctor has given a long list_of drugs.
The pharmacy of the
If has no drugs any way.
govt, hospital is closed.
a medical shop in her urgency.
The cost of the
on.
The night is fall
She retraces her steps with great
She wants to get back.
Nothing to offer,
disappointment.
So she goes to
drugs is very high.
She does not have even a ruppee to purchase them.
ing
At last the
only on payment.
the dehydrated child dies of
The film was terminated at this point.
simple diarrhoea.
The telling commentary ;
Well shop,
this is a telling commentary on the way the health
care delivery system (HCDS)
in our rural country functions.
The
participants devided themselves to discuss the story till that point
The discussion brought out several points which were
in 4 groups.
put to the audience by the group leaders - £ each group.
The social and economic conditions prevailing in the rural
areas were obviously scewed unfavourably to her and her village.
This is evident by the lack of roads nearby and lack of transport
facilities.
While everybody is aware of her and her child's problem, no
one tells to her anytime anything which may help her.
The manthrika
or Mantravadi has only primitive knowledge of medicine and treatment.
He does not know what oral rehydration therapy is.
There is no dadi or village nurse or auxillarly nurse midewife
seen anywhere in the
picture.
The situation is typical.
The general practitioner has given an injection to the child &
nothing more.
What for?
do for diarrhoea?
ing,
breast feeding
ignorance?
What has a single intramuscular injection to
He has not given to her any information about feed
No oral medicine.
or O.R.T. etc.
Is it incompetence or indifference?
Why?
Is it
Or the concern is to
get some money first, without any consideration about what proper
treatment could be given.
'
....4.
4
Accessible primary health facilities are not absent.
Suppo
sedly accessible tertiary facilities of a government hospital is not
there for a village lady.
She has neither the support of the husband
nor (or because of it) of the villagers.'
Inaccessibility apart, nothing moves in government hospitals
without payment.
The timing,
duty hours,' closure are all important,
the patient is of secondary importance.
transport and then in the bribing.
A lot of money is lost-in
Where will money be left to get
the drugs?
Leave alone money.
Where are the drugs? The Govt
*
hospital
supply has either run out or made 'inaccessible or has been freczed
for "other routes".
The local chemist has the drug, not the hospital;
It is not difficult to know why the govt hospital cannot provide *
any
In cities., on the other hand even the govt hospitals are better
provided with drugs,
but not in the rural area.
it is a long list. What is
Coming to the prescription;
essential/honessential?
If non essential drugs are there why were
Amongst the essential, could there be prioritisation.
they prescribed?
Are we sure about the.quality and reliability of all the drugs
available with local chemist?
'
..
Patients, cannot make choice regarding, medicines.
doctors must take more responsibility.
to explain-what is what,
it, how to take
it?
doctors ignorance?
Hence the
They did not take any paints
how to.take, what does it do, when to take
Why was the child not admitted?
Was_.it aga'in
Or indifference?
It was quite obvions that the child needed admission to the
hospital badly and was.in need of emergency IV transfusions;
The
doctor does not look like having appreciated the level of the serious
ness.
There, is no hurry, no body seems perturbed by the fact that
there is a dying child.
Is. it professionalism?
respect to human life because of it?
incompetence again?
Or is it scant
Or worse, is it ignorance and
Much worse - is it a trick tried upon the
patients mother to see if she wants an admission badly so as to make
it an additional point:to bargain for fees?
The education- curative and preventive;
Noone has properly explained in the film how diarrhoea occurs,
what are the effects, how it should not be allowed to worsen,
drugs act what is ORT and so on.
to be given not only to Ramakka but also to the Mantravadi,
doctor, villagers.
how
At every step education was needed
the local
But this is how it is in the reality.
The doctors also need to
be educated in social,
economic problems and situations of people.
education should be reoriented.
cultural,
For this the medical’
5
a
5
The mother continuing the breast feeding was a positive point
to be encouraged.
The role of pharmacist vis-a-vis a long prescription by a
doctor came in for discussion.
Does the pharmacist have the right
to modify delate, prioritise from amongst the medicines given to a
patient in case if the patient can bug only a few medicines and not
all?
The pharmacists present were of the opinion that they have no
be are to give our the
such right in a situation of this kind.
drugs and explain how it is to be taken or prepared.
decide, priorities modify,
delete,
Patient may
a long prescription and buy a few.
But that is a different thing.
; One ; Doctors should be more aware of
This raises 4 issues
rational therapeutics avoiding unnecessary and .Inessential drugs,
combinations that are not rational; where cheaper substitutes of
equal quality are available,
to use them; use cost as an equally
inportant criterion in selection of medicines prescribed and so an
and so forth.
This situation is absent today. Hardly any rational
therapentic criterion are followed while prescribing tonics,
expectorants,
antidiarrhoeals and so on.
endure under this horrible conglomerate of drugs,
The second issue is
abolished in most places.
called prescriptions.
; The institution of pharamacy as such is
General practitioners now-a-days do not
prepare mixtures and medicines.
below the dignity.
vitamins,
Thus pharmacists have to
Consultants treat it as something
Small and large privat : hospitals are abolishing
their "Drug stores" and "pharmacies1, and are found to be "prescribing
out".
The reasons for this is that they cannot men and manage it.
The terrible out come is
stepped in,
chat an extraordinary cost increase has
squeezing the poor and lower economical state people
The Government hospitals and medical colleges have it as a
badly.
lip service but are on the weaning side. The pharamacy and the pharma
cist have no leading role, worth any pride.
Third another interaction which should exist but has never
done so,
in most parts of our country,
including medical colleges,
the monitoring of indoor patient drug treatment.
is
This includes
judging rationality, preventing drug co-prescription with antagonism,
interaction or nullying of effect,
overdose, underdose,
bioavailability, pharmacokinetics etc.,
bioassay,
This is a very live and
important aspect of drug treatment in a western hospital,
brought to India.
need to be
In west this sort of help is available promptly
over telephones.
The 4th problem is revival of pharmacy,
the functions of which
are taken over by drug companies with their fixed dose channels. This
losses flexibility of dosages,
and accuracy- makes multiplicity of
.... .6.
6
medicine a cumbersome carriage and so on.
More than anything it is
the cost escalation out of it, which calls for revival of the
institution of the pharmacy.
(This discussion has been added at
this point from a talk given by Dr.Dubey later in the workshop.
was "soliced in-here” as relevent.
It
Dr.Dubey is from J.S.S.Pharamacy
college Mysore)
The resumption of
the slide show brought in reactions of
sales representatives,
chemists, doctors,
other health professionals
- All put together it can be summed up as : What can I do?
are like that.
Things
It is not my job alone to doll
I am not responsible.
That is how we stand to-day.
The Pain killers were briefly considered as a case study, by
II
enumerating different painkillers,
and comparing various issues.
anti-inflammatory drugs available
A handout prepared by Drug Action
Forcum-Karnatak on analgin was circulated by D A F-K.
The Afternoon session began by taking 4 pegs around which
Ill
analysis of the drug situation could be discussed.
The participants
again divided themselves in their original 4 groups.
The 4 pegs were:
I Formulations, as fixed does combinations with additives,
flavours, etc.
.
- II Tonics-as basically inessential drugs.
Ill Misuse or overuse of drugs and,
IV
Irrational Prescription and treatments.
Following are combined inferences of discussions of all 4 groups.
The Formulations ;
They have improved the drug delivery system,
can be harmful.
but the additives
The formulations are governed by pharmacopoea and
many ’will be found to
confirm to desired concentrations.
It was pointed out that only fixed dose combinations are
approved by WHO.
Whereas we have 65000 or more fixed dose combinat
ions currently circulating in market.
Tonics
;
Is it not irrational?
It was found that defining tonic was not easy.
mean improving tone? Tone
of what?
Muscle? Mind?
Is it getting energising feeling on drinking it?
cological utility?
of placebo?
Does tonic
Toning what?
What is the pharma
Does body need it, to be toned? Is it equivalent
What exactly is that Tonic?
The common point agreed was
that they are liquid and will generally contain alcohol vitamins,
semetimes iron, vegetable or animal protein extracts sometimes.
This appears to be a very vital differentiation; This also makes it
apparent that in gradients of tonics are not bad,
tion is wasteful and costly,
not necessar .
For this,
exept that,
combina
as most of the many drugs included are
better food with nutritions elements,
heme is preferable to tonic.
at
7
Seme of the ayurvedic physicians said that of the ayurvedic
"tonics", quite a few are useless or unnecessary.
raised by an MBBS.,
One objection
to any ayurvecic drug is the general lack of
standardisation making any rational comparisons incorrect.
Tonics die. not seem to have much except to fill up the demand
of p-atients through a
lacebo.
Differentiation of tonics from syrups, which are useful for
children is important.
It was pointed out that tonics have been classified as
"unnecessary drugs" by Government of India itself.
One form of missuse of tonics was that patients use some old
prescription and/or do self medication.
drugs,
Chemists freely prescribe
for ailments, without prescriptions,
examination etc.,
Steroid misuse by doctors is common and it is dangerous.
Major,
important end restricted drugs like tranquilisers,
sedatives are a commonly available commodity "Over the counter" so
are anti diarrhoeals,
expectorants.
It is difficult to controll all this misuse,
bad practises
etc., by law, because people are having a cavalier approach and the
The legal provisions are inadequate.
lae enforcement is difficult.
The legal machinery7 defficient in many ways and on account of failure,
or implementational failure.
Amongst professionals,
there is no updating,
refreshers courses, no reexaminations,
ing trends,
and its harms also.
no reorientation,
no truthful knowledge of chang
All they learn is,
at the hands of
medical representatives.
Irrational Prescriptions ;
The common examples of it are :
Long prescriptions with vitamins, tonics, which are not needed,
and are often prescribed under two brand names.
—
Cough syrups with expectorants and cough sedatives together.
—
Prescribing of doses, beyond the requirement and pharmacologi
cal limits, causing more side effects.
Shot gun therapies with multiple antibiotics, anti inflamatory
drugs and steroids, shot gun antidiarrhoeals.
—
Use of unnecessary antibiotics in inadequate doses and
durations,
The Hath! committee report was discussed at this point.
maj or recommendations of this report were ;
The
- Setting up a National Drug Authority.
- Nationalisation of multinational drug companies.
- Adoption of essential drugs list of 116 drugs on a national
level.
- Use of generic names instead of brand names.
... .8.
8
It was pointed out that in our present situation the require
ment of bulk drug is much higher than the production undertaken.
We
must concentrate on this
to improve the situation.
drugs is Rs.3000/- crores,
likely to reach a 5000 crores mark soon.
But this
high volume
markup,
like tonics etc.,
The sale cf
a lot of drug has a very high
is because,
though they are quite unnecessary for the
If was pointed out that only a few multi
needs of our poor people.
nationals are controlling this market.
*
-k
-k
*
THE BANNED & THE BANNABLE DRUGS
The discussion was then carried on,
;
on these two issues.
The
participants were apprised of what these drugs are, what is the class!'
It was also shown that although some cate
fication, categories etc.
others are not.
gories are compiled with,
A brief history of the
struggle against drugs like EP Forte was presented.
E.P.Forte is
high dose estrogen progesterbn combination.
a mixture of
It is
If menstrual bleeding does not follow the
used as a pregnancy test.
ingestion of E.P.Forte in 10 days, pregnancy is concluded.
case a pregnancy was existing when the drug was taken,
harmful to the foetus, scientifically speaking.
But in
the drug is
Yet Gynaecologists
in Delhi public trial frankly stated that it is not so.
It was later
revealed that they were pocketed by
drug industry,
the perpetuation of this sale, which,
inspite of a ban still continues
to be sold.
concerned with
This shows the dominance of the drug industry over the
professionals, market and the Federal Drug Controllers also.
Rational therapy and therapeutics was then elaborated upon.
This speaks of preferably no drug or else a minimum number,
essential ones,
in adequate does,
duration,
of only
combination, without
adverse mutual interaction.
*
*
*
*
The concluding remarks were made by Dr. S.K.Kelkar:
The organisers,
facilitators,
resource persons and students
who have gone through the workshop have raised issues for everyone
concerned especially for young medicoes to develop better practises.
We have opened a window and given a glance of the vast field needing
rectification,
for the sake of people and nation.
It is hoped that
greater insights are developed by students and practitioners for a
better situation, to be created by our efforts in future.
*
*
*
*
Appendices: 1), An article "Drug Culture" by Dr.Sanjeevanee, Ashwini
Hospital Madikeri, appended will demonstrate the whole
situation in a nutshell.
2)
Banned/Bannable drugs list is also appended to this
repo rt-cum-booklet.
9
The Mysore medical college boys have circulated,
in the city
of Mysore to all practitioners and chemists banned drug list and
intend to cover the whole district and follow it up, with visits to
all,
as a fall out of this workshop.
DRUG
CULTURE
Dr. SANJEEVANEE KELKAR
ASHWINI HOSPITAL,
MADIKERI.
Culture is the sum total of phenomena, habits,
reflexes,
psyche of a mass of people coming into existence, by long
cohabitati on.
drug producing agencies and the drug consum
In case of drugs,
ing population have existed long enough,
for this phenomenon to occur.
This briefly is the drug culture as it exists today and it is chang
ing further with the advances of medicine.
Another thing drugs have
done is it has also affected the culture of our society in bad ways.
n
• (Hu
Birth follows life which is followed by death. Any thing that
and the cycle is repeated.
is born is going to die,
These facts were
recognised and very well accepted and respected by our society
Since British rule started in India gradually this
centuries ago.
concept started changing.
Previously death was considered a natural
phenomenon and there was respect for death.
With developing modern
and more modern technologies and drugs, slowly man has a desire to
conquer over death.
Death is feared about and it has become face
less now.
Before the discovery of aspirin in 1899 the best medicine for
A
A
any disease was doctor's touch-which was considered a healing touch
and many diseases were treated with this faith,
Though the effect was
drugs.
’nocibo" effect of today,
as there were no
"Placebo it was never harmful than the
Iatrogenesis means all bad reactions produ
ced in the patients body and mind on account of the intervention of
the doctor,
investigation,
treatment etc.
This is the steeply rising
phenomenon of today’s .Allopathic medical practise.
Slowly the
"healing touch" of doctor's hand was forgotten and its place is taken
over by a pill.
killing paid
What harm has it done?
was the greatest sense,
animals.
Pain killing pill alongwith
killed the tolerence - the threshold for pain, which
only present in man.
Tolerance is absent in
Today people have starred looking at normal pregnancy as a
disease and
There
are demanding for painless labour.
was a misunderstood and misinterpreted religious faith
in our population,
that when you take a dip in the holy Ganga river
all your sins are washed out and
you become pavitra (Holy or sinless)
Such an easy answer for washing out your sins has permitted the people
....10.
1C
to behave wrongly,
I s
allowed them to commit crimes or behave immorally.
People are not afraid to do the sins.
in case of drugs also.
Similar thing is applicable
You don't pay attention to maintain your
health by positive active efforts,
abuse your body and then rush for
the drugs after getting diseasei
What public wanted they got it ;
There is a "pill for every
Slowly the number of drugs started increasing and new
ill" *
today
syrups and injections and spansules and tabsules & capsules
tablets,
and powders and baby foods and so on.
In 14 years 1948 to 1962,
4300
drugs were available in the American market only - and today in India
we have nearly 50,000 drug formulations marketed under different
brand names.
With more drug availability made possible,
and patients have adjusted to it,
prescribing doctors
and along with commercialization,
people have started depending more and more on drugs.
Antibiotics and vaccinations have done a great job and have
4
decreased morbidity and. mortality due to so many diseases and increased
the life expectancy.
But still the paradox is increasing.
of the society becoming "disease-free"
Instead
it is becoming "drug saturated"
and more number of drugs for less number of diseases has become
counter-productive to the aim of drug research.
Why so?
How many
agencies are there through whom a drug reaches to the ultimate
There are about 6 agencies:-
consumer?
1st - Government
2nd - F D A & Drug Controller.
3rd - Organisations representing the drug producers like OPPA,
IDMA, IDPL, etc.,
4th - Pharmacist / Chemist
5th - Prescribing doctor (instrumental consumer)
6th - Patients (True consumer)
About Government ;
Unfortunately the model ministry of drugs is the ministry of
(1 & 2)
Chemicals and not that of health.
Therefore drug production is
percived more as a chemical industry rather than as a health issue.
How competantly this ministry functions,
revealed by a recent example.
I think is very well
As every- bddy very well knows the
mishap - in 1986 January there were deaths in JJ Hospital Bombay because of consumption of glycerol which was laced with Dyethylene
glyool - a chemical used for industrial purpose.
The commission of
inquiries headed by Justice B.Lentin have revealed the nexus between
the pharmaceutical concern, FDA and also the government approved drug
testing labs the industries department and the government constituted
canmittees,
FDA
responsible for government hospital drug purchase.
has failed to order prosecution against 582 grossly erring drug
companies,
substandard drugs have not been withdraw out.
are Parke-Devis, Rapiakos,
WOKHARTD,
RCSCHE,
HAL, RALLIS, KHANDELWAL,
SADLLA, SK&F,
HIMALAYA,
others, who have manufactured then.
ALEMBIC,
These names
RANBAXY, SARABHAI,
ELYS and so many
.
.
11
The highest punishment for these merchants of death is a mere
issue of a warning.
(3)
Drue Industry ; Having lot of monetory power - multinational
strong representation in govern
drug companies have secured (MNDCS)
ment decision making and type and quality of drug production.
Consumer organisations are very small, work on a shoestring budget
& are ignored in decision making for their own interests and health.
Doctors are in drug industry's pocket.
Somebody says,
Industry's Instrumental consumer is doctor.
So before one becomes
a Medical graduate Drug company tries to form prescribing habits of
the doctors very hard, by meeting them,
Doctors are bombarded with grants,
pens etc.,
arranging lectures for them,
research, handbooks,etc.,
slide and film shows,
gifts,
Students and
stethoscopes, hammers,
Drug advertisements dominate the budgets and pages of
medical journals.
Drug Industry sponsers millions of Dollars in research in
Universities,
in Medical Colleges and Pvt. Labs and it greatly
influences the priorities of medical research.
Scientific data
So efforts are duplicated,
becomes trade secret.
results are not
shared and delays recognition of serious side effects that would
have been apparent from pooled data, much earlier.
Academic institutions depending heavily on drug company resear
ch money are reluctant to challange the company's practises in their
hospitals for fear of jeoparadising this support and thus,
advance
ment of scientific knowledge is strongly shaped by the pharmaceutical
industries-power and goals.
The so called drug-literature given to doctors is minus side
effects,
contra-indications and their toxicities and is more likely
to be mis-used by doctors and quacks.
Where are WHO,
IMA,
ended respectively 200,
al combinations?.
and the Hathi committee?
156,
Who have recomm
116 essential drugs and few more ration
In India about 50,000 formulations are marketted
under different brand
names.
Qualitywise there is no difference
in Brand name drug from that of a generic name of a drug which is
sold under the chemical name of the active ingradient.
is a fleral decorative name in bold,
much higher cost. On a Brand name,
Brand name
under which it is sold,
at a
drug companies invest lots of
money in imprinting their brand names on doctors & then dominates
in sell even after the patent is over,
over,
after a period of few years
same generic named different companies cheaper products.
By adding few ingradients which are inessential,
ses and name changes, but quality?
needs drugs for TB,
cost increa
Majority of poor population
lepracy or vaccinations.
They are under-produc
ed because this population does not have money to buy them. Production
12.
12
of vit B-complex 5.5% (TOP)
in all irrational combinations are
consumed in large numbers.
Vit A is much more needed has only 0.3%
TDP which is less than Vit K production in 1979-80.
production (TDP)
Total drug
in India was worth Rs.1260/- crores in which essen
tial and life saving drugs products were Rs. 350/- crores only. Multi
nationals in Indian drug industry have hardly any positive role to
play.
Through profit-making and monopolistic form of competition of
product differentiation through brand name,
advertising etc. these
companies are primarily interested in formulations for the well to
do at the expense of essential
drugs for the vast majority.
Medical
representatives can be best described by calling them, walking advert
isements on which companies invest very heavily.
(4)
Chemist is a small replica of the Pharmaceutical Industry.
Faults are so many.
Not keeping qualified pharmacist, so unknow
ingly selling wrong drugs is a frequent phenomenon.
Substituting
the drugs on their own, selling drugs without prescription, not
giving receipt,
selling take drugs to patients, selling samples and
selling sedative and harmone
expired drugs to illiterate patients,
ccmbinations-over the counter are commonly induged misdeeds.
Getting
and selling drugs from-government hospital supply and selling them
to same patients who have right to get them free is shamelessly
carried out aim-maximisation of profits.
In fact,
drug use in modem scientific medical practice has
practically been reduced to quackery though medical profession from
time to time speaks against quackery.
Why the ignorant,
uneducated,
unscrupulous non-medical profession since the very beginning?
it be allowed to continue?
Will
Is it not high time that the profession
starts dictating terms to the industry?
(5)
Doctors;
They are the instrumental consumers and most import
ant and most difficult to change their prescribing habits.
They
believe medical representatives information and so called literature
of drug companies fully.
Over-prescribing and forgetting the
principle of "Above all do not harm" and produce a condition more
serious
than the disease itself by giving drug which is not
necessary.
aue-asthesia,
Doing unnecessary operations, -ceasereans, misuse of
injection practices,
tonic misuse for keeping patients
good-will instead of educating him, steroid misuse,
any service - are all forms of malpractice.
taking bribe in
Doctor producing medical
colleges have become markets where degree is sellable and purchas
able, with what out come of what gravity? Is it Humane?
Medical profession which should be proud of its past notable
contribution towards human welfare,
has become a robot in the hands
of pharmaceutical industries and it has become a dis-honourable
money
spinning business.
Un-aware consumer : No body is permanently a patient and any
....13.
13 ::
This way every body is a consumer.
body can become a patient any time.
We have forgotten that health is not only every ones right but every
ones responsibility also.
Today we are born in a hospital and we die
in a hospital and in life Mediclaim, Health Insurance like companies
attach on us.
There is medicalisation of the society.
science is dictating the society to-day me *
e
People have blind faith in medicines.
political power.
it will do magic to them.
Medical
than religious or
They think
They look at ehri body as a machine.
Remedies?; Ultimately it has become a game of "Khokho".
Doctors comment upon MNDC, Companies criticise that doctors prescribe,
government does not make rules strictly and government is criticised,
government criticises drug companies and doctors,
company says we
work according to our owner where is our fault?
In this Khokho-
inspite of Khol nobody gets up.
Why we have become so thick skinned?
The sufferer has to revolt.
9
A
Who has to make the sufferer
aware? Government Staff and Private Nursing Home Staff will not do it.
Their interests are diffent.
As voluntary organisations we must see
what we can do for them and what we should do as our basic responsi
bility.
How? ;-
1.
By accepting an essential drug list for our practice in which
the cost would be an important criteria in selection in addi
tion to
efficacy and safety and Quality.
By demanding govern
ment to adopt a National drug policy aiming to provide all
essential
and life saving drugs to indigent people by impos
ing social control over drug industry and trade.
2.
W
a
By accepting generic prescribing rather than by brand name
products.
3.
By prescribing drugs which are Indian rather than foreign
government rather than private, small scale rational and co
operative sector rather than large scale sector.
4.
By stopping prescribing drugs whose only additional advertised
values are cosmatic embellishment,
combination,
immitative drugs,
elegant packing,
irrational
inadequate evidence of greater
value of products which are not properly evaluated.
5.
6.
By stopping the tonic practice.
By stopping prescribing when not needed just to retain the
patients goodwill.
7.
By adopting a rational drug theraphy which is necessary,
efficient,
8.
low cost and easy to administer.
By not. accepting physicians samples and other forms of induce
ments from medical companies.
catch on it".
"No donation is without a
It produces chain reactions.
....14.
14
9.
By propogating simple home remedies and locally available
herbal medicines after studying their efficacy.
10.
By adopting a more open policy of enquiry & use of traditional
medicine and non drug therapies.
11.
By supporting voluntary agencies active in drug field VHAI AND
AIDAN like organisations view points openly and actively and
helping the consumer.
America is healthy not because of more drugs and sophistication
but because nobody is poor there.
There more people die because
of drug reactions then road accidents.
For our health we have to think of priorities,
Clean water before antibiotics.
Food before Vit-pills.
Vacoination before kidney mechines.
Mothers milk before powdered baby foods.
Health for villagers and slums before more hospitals for the
affluent suburbs of capital cities.
In spite of our preoccupation with drug prescribing policy could
we commit ourselves to other more important health care priorities
and rethink,
about the old samskrit shloka
i.e. less drug the better.
15.
- 15 BANNED/BANNABLE DRUG LISTS
(A)
Fixed Dose combinations to be weeded out immediately as
recommended by the Drug Consultative committee GOI-Adapted,
modified by Dr. S.K.Kelker.
REAS ONS;
COMBINATIONS
i)
ii)
Steroids with any other drugs
Amidopyrine with
other drugs
1) Causes Adrenal Suppression
2)
Leads to adrenal insuffici
ency if withdrawn suddenly
3)
Interferes with immune mech
anisms which is dangerous.
1)
High incidence bone marrow
failure, with amidopyrine
2) Agranulocytosis, selectively
iii)
Chloramphenicol with Other
drugs
1)
Bone marrow aplasia with
100% fatality.
2)
Pancytopenia
3) Facilities to monitor blood
changes poor in country.
iv)
Ergot with other drugs
1) No rational basis
2) May cause severe bleeding,
periferal vasculopathy etc.
v)
vi)
Vitamins with antiinflamnatory
agents. Tranquilis er
Atropine with analgesics
antipyretics
1)
Actions in no way connected
synergistic, additive or
corrective as such, utterly
irrational.
2)
Cost escalation,
for.
1) May block sweating response
and interfere with reduction
of fever.
2)
vii)
Analgin/Metamizole with any
other drugs
uncalled
Myriad reactions due to para
sympathetic blockade, tachy
cardia, anginal pain,
glaucoma etc.
1) Analgin causes agranuloytosis
2) Whether with vitamins,
has no rationale.
it .
3) Restricted use in only inject
able "may be" permitted,
singly.
4) Combination with antispas
modics has no rationale,
because stoppage of spasm
stops pain for which analgin
is unnecessary and toxic.
viii) Yohimbine and Strychnine
with other drugs.
1)
Yohimbine cause CNS excita
tion, tremors.
2) Circulatory sympathetic
overdrive.
3)
No proven aphrodisiac nor any
other therapeutic value.
4) Strychnine may cause convul
sion tetanus like state.
5) No rationale available for
combining it.
6) Narrow therapeutic safety.
....16.
16
ix)
Iron, Strychnine,
Arsenic
1) Greater Therapeutic effect nil
2) Arsenic can cause accumulated
toxicity
3)
x)
Sodium Bromide and Chloral
hydrate with other drugs
No rationale.
1) Na, Br, and chloral and obsolete
as hypnotics, with greater thera
peutic safety, are available.
2) Narrow therapeutic safety.
xi) Tetrayclincs,
vitamin C.
Analgin, with
1) No additive effect
2) No rationale
3) Cost escalates without benefit
as vitamin 12
3
C' is costly.
xii)Ayurvedic Drugs with
stilboesterol and/or
other allopathic drugs
1)
Evidence of safety, safety of
interaction between the two,
not available.
2) Dangerous additive reactions,not
adequately proven,as not existing
3)
Approach to cause and cure bet
ween two systems different,
no common ground.
xiii)Phenacetin with other drugs
1)
Phenacetin considered actively
for banning for Methemoglobinemia,
hemolytic anaemia.
xiv)Chloramphenicol and strept
omycin
1)
Potential bone marrow toxicity
of chloramphenicol and its
remarkable action in Typhoid
should give it a status of
reserve drug and, should not be
used for common, often self
limiting diarrhoeas.
xv)
Penicillins with Streptomycin 1) Therapeutic flexibility of indi
vidual doses, a valuable charact
eristic of penicillins is
unaffordably lost.
xvi)M«re than one antihistaminics
1)
Difference in action is marginal
hence no additive action.
2) Additive hyphotic and other side
effects may make it troublesome.
3) Unnecessary Chemical load.
FIXED DOSE COMBINATIONS TO BE WEEDED OUT OVER A PERIOD OF SPECIFIED
TIME
i)
Antihistaminics and Antidiarr-1)
hoeals
In diarrohoeas there is no
allergic component.
2) Sedative action is not solicitated for every patient.
ii)
3)
Penicillins with Sulfonamides 1)
Bizarre side effects quite likely.
Irrational because a bacteriost
atic Sulfa and bactericidal Peni
cillin may cause intereference
with each others action allowing
only bacteristasis stressing the
immune response.
(Historically when these were
the only antibiotics or chemo
therapy agents available hence
they were initially combined
for meningitis but now
irrational).
2) Risk of bacterial
both drug.
resistance to
....17.
17
iii)
Antihistaminics (with pron 1) May cause dangerous additive
sedation; interfering with the
ounced sedative action)
sensoriurn of a sick patient
with sedatives
causing difficulty in evalua
tion of status; with dull mind
and not allow day today funct
ion nroperly, slowing reflex
acti,ity.
iv) Tranquilisers, antihistaminicsl)
and analgesics
Unwanted sedation in excess
interfering with activity,
reflexes and difficulty in
evaluation of pain, for
treatment.
2) Clinical situation demanding all
three drugs rare, does not
justify this.
v)
Analgesics and vitamins
3)
Thus unnecessary drugging is
caused which harms.
1)
Vitamins have no role in
analgesia
2)
Analgesics do not potentiate,
vitamins action, thus no
rationale,
3) Cost escalation.
vi) Paracetamol with anti
histaminics and
tranquilisers
vii)
Vitamins and anti TB drugs
1)
Additive high sedation effect
unwarranted.
2)
All simple .pains have no allergic
component, most pains with or
without allergic component, do
not need sedation.
3)
Chemical load high,
both undesirable.
cost high
) The only rational combination is
INH with pyridoxine as neuritis
is a dose depended phenomenon.
2) Unless dietary inadequacies
cause deficiency vita in need
not be prescribed.
VOLUNTARY HEALTH ASSOCIATION OE INDIA
40, INSTITUTIONAL AREA,
SOUTH OF I.I.T.,
NEW DELHI-J110016
Phones: 668071, 668072, 665018 □ Grams : "VOLHEALTH" New Delhi-110016
The Drag Itssue anadl the National Drag Policy!
Health, Development and Politics
Mira Shiva
India’s National Drug Policy is in the offing. While different
sectors in the drug industry are busy lobbying for concessions and
greater profit margins, the voice of the consumer is conspicuous by its
absence. Unused to participating in decision making or even analys
ing and expressing their views regarding health and drug policies, the
academic and professional medical bodies and faculties of medical
colleges and the health professionals have very little to contribute.
Hence, the medical establishment is not in a position to safeguard the
health of the people, or even meet their real health needs. Expecting
it to fight on behalf of the people for a rational health and drug
policy is being unrealistic.
More tragic has been the non-role of the social action groups,
many of whom have tended to look upon health work as a ‘relief’
and ‘reformatory’ activity, and health issues as non-political. Trade
unions, while being concerned about wages and bonuses, have not
cared to prevent the exploitation of their workers and their meagre
resources by the increasing commercialisation and pharmaoeuticalisation of health care. One would have expected protest against a drug
policy which is not in the interest of the people to come from some
such groups as also expect their support for such a protest made by
others.
It is crucial that people in all walks of life understand the drug
situation in the country, the implications of the policy changes being
formulated and their impact on their own life and that of other
people.
Need of a rational drug policy
A Drug Policy is an integral part of a health policy. The increas
ing dependence on drugs and medical technologies has successfully
Dr Mira Shiva works with the Voluntary Health Association of India,
New Delhi as Co-ordinator of Low Cost Drugs and Rational Therapeutics and
i". Convener of the All India Drug Action Network.
1
managed to create ‘myths’ and "beliefs’ that there are medical
solutions to problems arising out of unmet basic needs, solutions for
which can only come from socio-economic and political changes. With
the proliferation of health personnel (trained and untrained) chemist
and druggist shops even in remote rural areas, the ‘pill culture’has
infiltrated wide areas and communities through ‘commercial’ channels.
Misguided yet genuine efforts of development workers to ensure that
the poor villagers get a share in the fruits of‘modern science’have
converted millions into consumers with artificial drug needs. This hac
created profitable markets for the drug industry. Unfortunately, the
controls that should have gone with this development, such as the
availability of unbiased drug information, the warnings etc-, are
woefully missing.
Increasing dependence on drugs is not merely marginalising
and systematically eroding the previously existing alternatives, many
of which were holistic, low cost, locally available, indigenous and en
couraged self-reliance, but most of the drug consumption is for self
limiting trivial health problems. Local alternatives for these have
existed and are still often the most appropriate and safe. For people’s
health rights to become real, a drug policy has to remove this artificial
dependence. A drug policy can complement health care efforts or
totally cripple them. We can choose an unhealthy dependence on the
medical-industrial complex in spite of its very little impact on the
health status of our people or we can ensure a rational use of drugs
which is in the best interest of the people.
WHO’s Action Programme for Essential Drugs has drawn up
guidelines for rational Drug Policies, which are therapeutically,
medically sound and economically speaking the most appropriate.
While most WHO programmes are given whole-hearted support,
where WHO’s Action Programme for Essential Drugs is concerned,
vested interests have blocked it from, being given the support it
deserves. Thus the drug industries seem to not merely dictate (subtly
brainwash) the prescription practices but also influence the drug
policies themselves. This indicates their increasing power and our
continuing lack of strong reaction.
India has one of the best developed pharmaceutical industries
in the Third World. According to UNIDO (Lisbon Conference),
India’s pharmaceutical industry was considered to belong to category
V, i.e. technologically developed enough to be totally self-reliant. Yet
many ills ail the pharmaceutical scene and some of the ills are critical.
India’s Pharmaceutical growth has been very impressive:
2
Table 1: India’s Pharmaceutical erowth 1932-53 to 1982-83
SI. No.
item
I.
2.
3.
Number of units
Investment
Bulk drug production
4.
5.
Formulation
Imports
6.
7.
8.
Exports
Price index of drugs
Price index of all
commodities
1953-54
1982-83
% increase
1643
Rs 24 crore
Rs 27 crore
(64-65)
Rs 35 crore
Rs 16 crore
(51-52)
Rs 0.8 crore
too (1970-71)
100 (1970-71)
6631
Rs 600 crore
Rs 325 crore
300
2,400
1.800
Rs 1 545 crore
Rs 141 crore
4,300
780
Rs 111 crore
167
287
13,700
67
187
Source: Gharpure 1985: 37.
In spite of this increase, drug production, distribution etc.
have not been in keeping with the health needs of our people. While
on the one hand shortages of essential and life saving drugs have
occurred, on the other even rural markets have been flooded with
costly brands of absolutely irrational drugs of doubtful therapeutic
drug needs.
Disparities between needs and production
For the majority suffering from ill health, their health problem
stems from unmet basic needs of adequate food, safe drinking water
and healthy working and living conditions. For the right to health to
be real, these issues have to be tackled. But in practice it does not
happen. As a result, the poor who have health problems because of
food deficiency (and low income) are ignored. While production and
availability of pulses has decreased, the so called high protein food
supplements like protein Complan, Bournvita are pushed vigourously
in the market (Gopalan 1985: 12-13). The average growth rate (height)
of rural children is far less than that of their urban counterparts.
There has been a deterioration in the growth of the poor Indian
children between 1957 and 1978. Food availability now is 10% lower
per capita, per year, than in 1953-54 (Gharpure 1985). According to
Dr. C. Gopalan (1985: 12-13), ‘only less than 3 million of the 23
million to be born in 1983 will become truly healthy, physically fit,
productive and intellectually capable citizens of this country.’ In
India we have over 30,000 children becoming blind each year (and
this is a gross under estimate) because of the failure to get vitamin A
in their diets (UNICEF 1984: 52). Children who are denied adequate
3
protein, calories, essential vitamins and minerals can in no way com
pensate for them by drinking costly tonics (Jayarao 1976).
Table 2: Targets and Production of Monitored Bulk Drugs
1980-81
target-prod
UNIT
Vit A MMU 66.0 59.8
1982-83
tar-prod
77.0 52.49
1981-82
tar-prod
66.6 52.6
1983-84
tar-prod
90 60 23
1984-85
tar-prod
105 55.9
Sourcts' Narayana 1984: 244; Govt, of India 1985: 38.
With the increase in total population and with 42 per cent of
our population below the age of 1 5 and about 50 per cent below the
poverty line and malnourished, requirements of Vit A are definately
much more than the production target (Table 2). While a tremendous
gap exists between the target and the estimated production of Vit.A
drugs in 1984-85, the production itself has gone down from 62.2
MMUin 1978-79 and 59.8 MMU in 1980-81 to an estimated 55.9
MMU in 1984-85. Given the increase in requirements, this depreciation
is significant
India has over 60 million people with endemic goitre. Iodine
deficiency in pregnant women and children is known to be associated
with birth of deaf, mute or mentally subnormal children. Over 1,000
significantly mentally deranged children are known to be born in the
Terai region alone. In areas with 50 per cent population with goitre,
2 per cent of all children born are expected to be abnormal (Gopalan
1985). All this is for want of iodized salt. We produce only 20 per cent
of our overall requirement of iodized salt, much of which does not
even reach the endemic areas (Nutrition Foundation of India 1983).
If such large numbers are allowed to be born underweight and
allowed to develop only to be blinded, maimed or mentally retarded—
with health and drug policies failing to change the situation signifi
cantly even after 38 years of independence—then the policies which
have already been found to be inappropriate need to be totally
revamped with relevant restructuring. The Government of India
(1982) itself has acknowledged that
the imported and inappropriate model of health services is
top heavy, over centralised, heavily curative in its approach,
urban and elite oriented, costly and dependency creating.
India has 10 million TB patients, out of which 2 5 million are
actively infectious and 50,000 die each year. We produce J of the
mininum requirement of anti-TB drugs, according to an 1CSSR-ICMR
Report. While the production rate of basic and cheaper anti-TB drugs
has tended to be inadequate, costly second line drugs have been
vigorously pushed. To cite some statistics, whereas the target for
4
♦
production of IN drugs which are cheaper, had gone up marginally
from 140 tons in 1981-82 to 158 tons in 1982-83, the target for costly
drugs like Ethambutol had shot up from 32 to 154 tons.
Non-essential drugs
75 per cent of the drugs in the market are non-essential and 25
per cent of our drug market constitutes of tonics, vitamins, tonic
restoratives, enzymes etc. (1CSSR-1CMR 1981). Tonic sales in India
is 12 per cent as against 3 per cent in developed countries. On the
other hand, sale of Vit.A is a mere 3 per cent. In 1977, an UNCTAD
study showed that 34 per cent of all drugs sold were tonics, tranquil
lisers and cough mixtures. Anti-microbials were a mere 2 per cent and
essentia! vaccines merely 7 per cent. In 1980, the production of
vitamins, tonics and cough and cold remedies amounted to 23.5 per
cent alone.
The Hathi Committee (Government of India 1975) drew up a list
of 116 essential drugs for India. The World Health Organisation (1977)
recommended around 200 essential drugs. Sweden has 2,000 drugs
in the market. India has 30,000 formulations out of which 5,000 are
useful and 2,500 of marginal use (Gharpure 1985: 326). 68 per cent of
the drugs are classified as obsolete by the DCC. Of these 30 per cent are
useless and 70 per cent are actually harmful. The best private hospitals
in U.S. possess their own hospital formularies with restricted drug
lists. U.K. has recently decided to restrict the drug list in the National
Health Services at a considerable saving to the Nation (as a recent
issue of the Guardian (1985) has brought out).
WHO has given very clear guidelines regarding selection of
essentia! drugs. It is upto the different countries to draw up their
own lists based on their priority health needs, to guide drug produc
tion, drug distribution, drug information, quality control etc. Fiscal
reasons have guided the entire policy formulation in our country,
much to our regret (Melrose 1983).
While WHO’s essential drug list has been considered extremely
short and arbitrary by the drug industry as well as our own drugs
control authorities, slashing the essential drug list under price control
from 343 to 95 merely to ensure that the prices of all other drugs are
decontrolled, does not make sense.
Continued sales of banned, bannable and hazardous drugs
It is amazing, how with imports of ‘high-technology’ rarely are
the ‘controls’ and 'precautions’ imported. Technological know-how
has come to mean know-how required for putting up the production
plant. The knowledge of the status of the product in terms of
5
efficacy, safety, comparison with other drugs, evaluation studies in
other parts of the world are all part of the know-how. Numerous
unsafe and controversial drugs continue to be marketed. For exam
ple, while 1.5 million Indian children die of diarrhoea each year,
the drug market of anti-diarrhoeal is flourishing. Message of ORT
is drawn in the glib sales talk for anti-diarrhoeal. Lomotil, known to
have caused several infant deaths due to its extremely narrow safety
margin, continues to be given to children (Medawar and Freeze
1983).
Clioquinols — Hydroxyquinolines (Mexaform like drugs): The
crippling and blinding of over 11,000 Japanese with mexaformenterovioform is well known. Efforts by drug companies involved
and the medical community to associate SMON Syndrome (Subacute
Myelo Optic Neuropathy) with ‘virus’ ‘genetic’ causes were
countered by a group of socially conscious lawyers, journalists and
doctors. Dr Olle Hansson showed the association of blindness
(optic neuritis) with mexaform and enterovioform and proved tnat
the drugs were absorbed from the gut and therefore could affect the
nervous system. Efforts of Japanese lawyers and SMON victims to
get these drugs withdrawn worldwide, succeeded in forcing CibaGeigy to withdraw Enterovioform and Mexaform from the world
market by March 1985.
Over 2,000 Mexicans died in 1972 in a Typhoid epidemic
because resistance to chlosamphenicol had emerged unrecognised by
the medical professionals. Emergence of resistance to a drug means
failure of the drug to destroy the harmful organisms. Typhoid resis
tant to chlosamphenicol in endemic form is being reported from
Kerala and is spreading. Resistance to majority of the commonly
used antibiotics contributed to the deaths of over 2,000 in 1983 in the
West Bengal dysentry epidemic. Besides death and uncontrolled
spread of diseases, resistance due to misuse of patent antibiotics for
trivial problems means dependence on costly, unaffordable alterna
tives.
The Thalidomide disaster sums up the problem of hazardous
drugs and their implications. Over 16,000 children were born mal
formed, invalid for life because their mothers consumed Thalido
mide—a drug which was sold to them for morning sickness. Obvi
ously tests on effect on foetus was not done.
The E.P. drug story is a medical crime story. In 1976 WHO
based reports from Australian Medical Association warned all the
member countries not to use high dose estrogen-progesterone drugs
for pregnancy testing—as it was found to be associated with foetal
malformation. But in India till 1982, drug marketing literature
6
advocated the drugs for pregnancy testing. What was probably most
horrendous was the fact that not merely were these drugs being pre
scribed freely by the doctors without any warning to the mothers, who
had every intention of having their (healthy) babies, but these
products were sold freely over the counter without warning to any
one, for the asking. The drugs were consumed not merely for preg
nancy testing but also for inducing abortion and several other gyneco
logical problems.
Warnings in medical jargon, warnings in an alien language,
warnings in microscopic letters, even if written would not have made
any sense to the women. But not even these legalities are observed
in all these harmful drugs. Following a national campaign against
the drug and public pressure demanding a ban, the drug was banned
by the Drug Controller of India. But some pharmaceutical compa
nies went to court and got a stay order against the ban. Thus they
continue to produce these and other harmful drugs and do not even
give the statutary warning—even in an alien language.
Inadequate drug control
Five quality control laboratories and 500 drug inspectors attempt to
maintain quality control of drugs produced from over 8.000 formulators and sold by several thousand drug distributors or chemists in the
entire country. The recommended ratio of drug inspectors to drug
units is 1:25 and of drug outlets is 1:100, but the numbers are grossly
inadequate. Japan which is considered to be the seat of small scale
industry has only 1,000 drug units. U.S. with 16 per cent of the drug
market has only 700 units. India with a mere 1 per cent of the
world market and 30-40,000 formulations, has 8,000 formulators.
Quality control is bound to suffer and lead to sales of substandard
and spurious drugs. 20 per cent of the drugs in the market are sub
standard as accepted in the Parliament.
Complaints and demands for increase in the number of quality
control labs for drug inspectors has been evaded by statements from
the authorities complaining of ‘financial constraints.’
There is no doubt that enough legal loopholes exist. These
ensure that almost each and every case fought by the government
authorities against the drug houses who flaunt the drug legislation is
either painfully prolonged or lost. In 1980 the drug consultative
committee reviewed 34 categories of drug combinations of which 23
categories were recommended for being weeded out with 16 catego
ries to be removed immediately. In 1981 the Drug Advisory Board
diluted the list by excluding some of the drugs from the list.
7
In July 1983, the Drug Controller of India issued a gazette
notification banning 22 categories of drugs. In 1984, several of these
drugs were available in the market. And today (in 1985), many of
the banned drugs are still available. Many doctors and chemists are
not even aware of the ban. Failure on the part of the Drug Control
authorities to make the banned brand drug list available and further
failure on their part to ensure withdrawal of these drugs from the
market has ensured confusion and non-application of the ban (Shiva
1984).
The result of such violation of the ban can be seen in the high
profit made by the multinational pharmaceutical companies that con
trol drug industry in the country. The following table indicates the
amount of profits taken out of the country in comparison with the
total capital investment made.
Table 3: Profit remittancesofsomemultination.il companies
Drug Company
Original capital
investment
Glaxo
Pfizer
Ciba-Geigy
Cynamid
Bayer
Rs 1.5 lakhs
Rs 2 lakhs
Rs 3 lakhs
Rs 1.50 lakhs
Rs 4 lakhs
Profits remitted to
parent company in 1980-81
Rs 135 lakhs
Rs 260.82 lakhs (in 3 yrs)
Rs 162.57 lakhs
Rs 157.64 lakhs
Rs 135.82 lakhs
The new drug policy
Unlike the Hathi Committee which systematically reviewed the
pros and cons of various aspects of the drug policy before coming
out with its recommendations, the report of the National Drugs and
Pharmaceutical Council is far from comprehensive. Drug pricing and
fiscal benefits seem to have guided the new drug policy. All other
crucial aspects of a rational Drug Policy have been totally neglected
Our criticism of the National Drug Policy is for the following rea
sons:
The drug policy document as proposed by the steering com
mittee is not based on the actual health needs of the people, the need
for self-reliance in the drug industry, weeding out irrational and
hazardous drugs and a distribution scheme to' make drugs availale to
the people at low cost. The policy document consists of only dis
cussions regarding the profitability and viability of the drug industry.
In fact the entire report reads as if it was a forum of bargaining and
investing concessions for various sectoral demands. It is interesting
that the policy document is framed by the government and the drug
8
industry without representation from the health sector, (governmental
and voluntary) consumer groups, trade unions and other mass
organisations and various scientific bodies. With a composition as
noted above, it is not surprising that the drug policy has been framed
in isolation from a rational health policy.
Surprisingly, a review of the aims and objectives of the drug
policy, 1978 and the DPOO 1979 in respect of the functioning of the
industry for the last 5 years, has not been made. In the absence
of such a review, we fail to understand how a new drug policy can
be formulated. The following glaring ommissions can be mentioned:
(i)
No effort in identifying and weeding out of drugs medi
cally accepted as hazardous.
No effort at drawing up a National Essential Drug list
to guide the production, distribution and prescription of
drugs.
(iii)
No effort to make available unbiased drug information to
health personnel and people.
(ii)
(iv)
No effort to evolve distribution schemes for drugs for
the people.
(v)
No effort to ensure ethical marketing and trade practices.
Even the points discussed by the Drug Steering Committee
do not have the interests of the people at large but have been based
on the needs of the drug companies. For instance, the demand
pattern computed by the Drug Steering Committee and the working
group are not based on the disease patterns existing and the conse
quent need for drugs but on simple extrapolation of existing skewed
demand patterns. No proposal has been made to ensure the produc
tion of essential drugs with appropriate price controls.
With regard to the pricing policy to be followed, the only con
sideration that the DSC has taken into account is the profitability of
the drug industry; The principle that the essential drugs should be
made available at low prices has been paid lip service but no attempt
has been made to formulate policies which will lead to such a result.
The DSC has decided to change the various categories of
drugs and the markups to be provided for each category. However,
no reason has been put forward why the price markups should be
changed The Government of India and BICP (Bureau of Industrial
Costing & Pricing) have yet to produce data that the markups allowed
are leading to losses in the drug industry.
The essential drug list has been reduced to 95 with no re
duction in the irrational and hazardous drugs in the market. Negative
and positive lists have been used elsewhere to guide the selection of
9
drugs in the market. Bangladesh (Rolt 1984) and Mozambique are
very good examples (Shiva 1983) But no such list has been prepared
in India. We have no National Formulary nor Therapeutic Guidelines.
Exclusion of certain basic drugs e g. chlorthiazide as a diuretic
phenobarb and phenytoin as anti-epileptics, pentaimidine for Kalazar,
etc., when 4 general anaestheticsand 4 anti-cancer drugs could be
included, must have required some rationale which we just cannot
find.
The Methodology used for working out demand estimates is
extremely irrational, based more on past warped production patterns
than actual health needs. Consequently, with increased profitability
and liberalisation, further flooding of the markets (rural and urban)
with irrational but more profitable drugs is expected. With most of
the other drugs outside the drug control basket, an increase in drug
prices is envisaged.
With absolutely no change in the number of hazardous drugs
in the market, no effort at ensuring unbiased drug information on
ethical marketing practices and no improvement in quality control
or drug legislation but with ensured profitability, the health of the
drug industry is going to be well taken care of. The tragedy is that
this increased profitably is at the cost of the consumers, their scarce
resources and their health. Refusal to swallow a pill, according
to Fritzof Capra, is a political action. Refusal to swallow pills which
are deleterious to healh is the beginning of an important process by
which people begin to reject not only pills but policies such as the one
in the offing.
REFERENCES
Gharpure, Y.H. ‘Drugs for Masses,' The Eastern Pharmacist, 26 (n. 326, Febru
ary 1985), pp 35-38.
Gopalan, C. Nutrition and Health Care: Problems and Policies. New Delhi:
Nutrition Foundation of India, 1985.
Govt, of India. Report of the Committee on Drugs and Pharmaceutical Indus
tries. New Delhi: Ministry of Petroleum and Chemicals, 1975.
10
------ . National Health Policy Document. New Delhi: Ministry of Health and
Family Welfare, 1982.
------ . Annual Report: 1984-85. New Delhi: Ministry of Chemicals and Fertili
sers, 1983.
ICSSR and ICMR. Health for All: An Alternative Strategy. Pune: Indian Insti
tute of Education, 1981.
Jayarao, Ramla. ‘Tonics: How much an Economic Waste?’ Af/c Bulletin
(November 1978).
Medawar, Charles and Barbara Freeze. Drug Diplomacy. London: Social Audit,
1983.
Melrose, Dianna, Bitter Pilis. Oxford: OXFAM, 1982.
Narayana, P.L. The Indian Pharmaceutical Industry: Problems and Prospects.
New Delhi. National Council of Applied Economic Research, 1985.
Nutrition Foundation of India. The National Goitre Control Programme—A
Blueprint for its Intensification (Scientific Report‘No. 1). New Delhi:
Nutrition Foundation of India, 1983.
Rolt, Francis. Pills, Policies and Politics. London: War on Want, 1984.
Shiva, Mira. People-Oriented Drug Policy: Mozambique Experience. (VHAI
Handout). New Delhi: Voluntary Health Association of India, 1983.
—----- . Banned, Banable and Hazardous Drugs. Paper Presented at the All India
Drug Action Network Meet, Wardha. New Delhi: Voluntary Health
Association of India, 1984.
UNICEF. An Analysis of the State of the Childrenin India. Geneva: UNICEF,
1984.
WHO. The Selection of Essentia! Drugs. Geneva: Technical Report Series No.
615, 1977.
Reprlated From
SOCIAL ACTION Vol. 35 No. 3 July-September 1985
11
Ijt ALL INDIA DRUG ACTION NETWORK
. V / J ' C-14 COMMUNITY CENTRE S. D. A. NEW DELHI 110016
RATIONAL DRUG POLICY - STATEMENT
AIDAN
All India Drug Action Network (AIDAN), is a forum, and
coordinating body of organizations, and individuals
all over the country working towards the adoption and
implementation of a people oriented Rational Drug Policy
in India as a part of a Peoples Health Policy. It sets out
the following outline for the Drug Policy :
Health
Policy
and .
drugs
Majority of the Indians suffer from the diseases of
poverty and iqnorance, i.e. communicable diseases,
diseases due to undernutrition etc. Most of these illnesses
are preventable and curable. In addition, distorted pattern
of industrialisation and urbanisation has led to the deve
lopment of so called diseases of industrialisation. What
we need most is adequate nutrition, adequate and safe
water, universal sanitation, development without damaging
environmental balance and primary medical service,
available to all.
Role
of
drugs
Although drugs constitute only a small part of the overall
health care, they are
most urgent, essential and
hence a priority need in the country where incidence
of death and disability from diseases is high. So long
the basic elements of health care are not made available
universally, medical care will continue to be the priority
service to reduce death and disability and in this context,
drugs understandably assume a vital and priority role.
Present
situation
The fact that drugs can save life and relieve sufferings
has been exploited by the drug industry, which is oriented
mainly to profit making, to push all sorts of irrational
druas onto the consumers. The druq industry and the
medical establishment has created a very drug-dependant
health culture which eclipses the much-needed sustainable
solutions to the real health problems. Doctors and nondoccors alike are made to believe that drugs are '’cure all"
-:2:for all ills. Health is still regarded as basically an indivi
dual or personal responsibility and not a product of
social factors.
It is also believed that freedom from diseases could be
obtained by better an- better and more and more drugs.
Such a belief among educated and illiterate alike, has
led
to a universal craze for drugs and
this DRUG
CULTURE has come to dominate the society. In this
situation it is not surprising that drugs provide an oppor
tunity for unlimited profit-making by the drug industry,
since hardly any consumer asks for the necessity, utility,
rationality, price-justifiability and harmful effects
of a drug. It is not even asked whether a substance sold
as Drug is actually a Drug at all. As a result, about 60%
of the drugs in the market are unscientific or harmful
or substandard; a large number of these are not actually
drugs; many drugs are consumed by those who do not
need it; people die or are disabled from the effects of
harmful drugs; drugs are sold at fantastically high prices;
and most serious of all life saving and essential drugs
are not available to the majority that need them most.
Even 38 years after independence, multinational corpo
rations continue to dominate the drug industry in India.
Further, the majority of their production consists of
drug formulations and not bulk drugs. Though, according
to UNIDO, India has the capacity to be self sufficient
in bulk drugs, we still import 40% of our bulk-drug
requirement.
Objectives
of the
Rational
drug
policy
We feel that the Rational Drug Policy objectives should
include the following :-
A.
ASSESSING THE DRUG-NEEDS
1)
To identify the drug needs in consonance with
the health needs of the people, particularly
those required for primary health care; to
prepare a graded essential and priority list of
drugs for different levels of health expertise
inkeeping with actual health needs of the people.
2)
To eliminate irrational, useless and hazardous
drugs.
-:3:B.
C.
PRODUCTION, PRICE AND QUALITY CONTROL
1)
To make all drugs available at low prices to the
people, particularly the essential & priority drugs.
2)
To ensure quality control of all drugs.
DRUG DISTRIBUTION
To establish a national corporation for the distri
bution of drugs; retailing of drugs through fair
price shops and government's health infrastru
ctures.
D.
E.
DRUG INFORMATION AND ETHICAL MARKETING
1)
To ensure a drug information system for health
personnel and consumers.
2)
To ensure ethical marketing.
3)
To abolish brand names and introduce generic
names for all drugs.
SELF - RELIANCE
1) To develop
self reliance in drug technology.
2) To foster and encourage the growth of the Indian
Sector and to provide a leadership role to the
public sector.
3)
F.
To aim at quick self sufficiency in the output of
drugs with a view to reducing the quantum of
imports.
RESEARCH AND DEVELOPMENT
To promote research and development for selfreliance and in accordance with the needs of
the Indian people.
G.
LEGISLATION AND ADMINISTRATION
1) To provide comprehensive drug legislation
and administrative support to deal effectively
with and implement all the above arms and
objectives.
-:4:-
2) To ensure smooth Centre-State relations anc
inter-departmentai coordination for effective
and relevant drug production, drug control and
drug supply.
H.
HUMANPOWER DEVELOPMENT
To fulfill the needs of the above Rational Drug
Policy, different type of technical personnel
(e.g. druggists, paramedics, etc.) need to be
adequately and appropriately trained in adequate
numbers.
A. ASSESSING THE DRUG NEEDS
A
1.
Identification of Drug needs and Prioritized Essential Drug List
i)
The National Drug Formulary should be revised and compiled
by an expert multi-disciplinary committee with suitable repre
sentation from all the sections of health professionals. This
committee should draw up the essential priority drug list,
keeping the following criteria in mind * Medico-social justification should act as a primary
criterion keeping in mind - efficacy, safety, cost, ease of
administration, potential for misuse, indigenous production.
* Priority for production has to be given to the drugs
required for diseases causing greater mortality (death),
greater morbidity (illness), severe sequelae (after effects).
* Drugs used in National Programmes e.g. TB, leprosy,
malaria, blindness, goitre control, and immunisation
programmes should get priority.
This list should be revised periodically.
ii)
Selection of the essential and priority drugs would be followed
by preparation of graded drug list for different categories of
health personnel and health institutions. These lists should
form the basic guidelines for bulk purchase procurement and
requisition stocking and dispensing.
-:5:-
An appropriate authority (see section G2) should continuously
assess drug needs and drug production and monitor capacity
utilisation in the industry, drug utilisation patterns, health
needs, changing pattern of diseases, drug requirements, new
information on old drugs, introduction of new drugs, efficacy
of the existing policy of production, distribution and use of
drugs.
A
2.
Withdrawal of hazardous, irrational and therapeutically useless
drugs.
i)
All the drugs in the market should be scrutinised to assess
their rationality on the basis of standard text books of medicine
and pharmacology. All drugs which are not recommended
in these text books should be banned. Those drugs which have
life-threatening or serious side-effects and for which equally
effective alternatives are available should be banned immedia
tely. The rest of these drugs should disappear from the market
within one year.
ii)
No fixed dose combination forms should be allowed to be
manufactured if an alternative single ingredient drug is available
for the purpose, which is therapeutically equivalent and more
cost effective.
iii)
Drug Legislation should be modified to ensure that no stay
order is granted in cases pertaining to banning hazardous
drugs in the interest of public health.
B. PRODUCTION, PRICE AND QUALITY CONTROL
B
1.
Production and Price Controls
i)
The priority drug list should be a part of much larger essential
drug list based on WHO recommendations as well as those
of our own National Drugs and Therapeutics Authority. In
this essential drug list, life saving drugs and drugs for primary
health care shall be put as category I termed as priority drug
list and the rest of the list shall be put in category II.
ii)
The production of essential drug formulations shall be a minimum
75% of total formulation turnover of each manufacturer now
and shall be brought up to 90% in five years. The priority
drugs shall constitute 60% of the above essential drugs and
shall be raised to 80% of the essential druns in the next 5
years. The above production quota should include all dosage
forms of essential and priority druqs.
B
iii)
All companies having equity above 26% shall be identified
as foreign companies (as per RBI definition).
iv)
All foreign companies shall produce bulk as to formulation
ratio of 1:5. For other companies the ratio shall be 1:10.
v)
A mechanism should be evolved to provide incentive to those
companies which produce more than the required quota of
essential/priority drugs and deterrent punishment to those
companies which produce less than the required quota as
given above.
vi)
The priority drugs should be made available at low prices.
If required, they may even be subsidized. Before any major
revision in the pricing policy is done, as a policy there should
be an independent study to assess the cost, profitability as
well as availability and price from the point of view of consu
mers. Profit-making should not be the sole basis of the drug
industry. All taxes from priority drugs should be abolished
to reduce the prices of such drugs.
vii)
The trade commission shall be fixed at 20%. However, this
is the total commission which will be paid from the principal
manufacturer to the distributors and the intermediaries. While
the markup under the head of trade commission will be increa
sed, the markup under the head of sales promotion will be
decreased for essential and priority drugs.
viii)
All drugs including nutritional supplements, except that produced
by small scale sector, shall be under price control.
ix)
The small scale sector can be free from price and production^'
controls. However, the small scale sector will be defined
as those companies whose turnover is less than 20 lakhs and
not linked to large scale and organised sector units through
ownership, financial participation or marketing arrangements.
2.
Proper Drug Registration and Monitoring
. Registration
1.
All pharmaceutical products, both ethical drugs and over-thecounter (OTC) preparations offered for sale should be duly
registered by a competent authority.
-:7:2.
Commercially sold indigenous medicines should also be registered
and pharmaceutical products which are not registered should
not be allowed to be marketed.
3.
Pharmaceutical manufacturers and traders must provide the
registration authority with a list of all countries in which the
specific product has not been accepted for registration.
4.
Pharmaceutical manufacturers and traders should inform the
registration authority if a pharmaceutical product already
registered in the country has been removed from the register
of any other country together with the reason 'for its removal.
5.
Pharmaceutical manufacturers and traders, when applying for
registration of a product, must be made to undertake that
subsequent to the product's registration, they will provide
the registration authority and consumers with all new informa
tions they receive about its effects, adverse reactions and
interactions.
6.
Central Drug Control authorities should have an up-to-date
information about the various drug formulations in the market,
their combinations, their date of licensing, drug information
being given with them by the producers and the latest inter
national medical views on the products.
7.
Drugs which have been banned from sale after being marketed
for some time in one country must not be submitted for clinical
trial or marketing in India. The onus of proving why such
a non-essential drug should be introduced or allowed to continue
in the market should be with the manufacturers.
8.
Whenever a new drug is tested on healthy human subjects
or on patients, the clinical trial must be authorised and moni
tored by a local "Ethical Committee" and must be carried
out only with the full informed consent of the people and
patients concerned.
Medical Audit System
It should be introduced to review the medical costs, the prescription
practices, patient complaints of negligence or financial exploitation
and drug misuse. At least minimal medical/clinical record keeping
should be made mandatory. Medical audit systems should be intro
duced in a systematic manner.
Physicians and pharmacists should be answerable to Rational Thera
peutics Committee of Experts. This could be appointed by Medical
Council or any other academic neutral body. Medical experts involved
in primary, secondary and tertiary medical care, chemists and
consumer organisations should be represented.
C. DRUG DISTRIBUTION
i)
A National Corporation for distribution of drugs and pharma
ceuticals to retail drug outlets, hospitals and dispensaries
should be established.
ii)
National Drugs and Therapeutics Authority (see section G2)
(or its sub-committee) should look into the drug needs of
the peripnerai health units to identify the bottle-necks and
deal with them as a priority and ensure timely drug supply.
iii)
This corporation should look into
- requirement estimation of various drugs and their dosage
forms;
- purchasing effective, safe and quality drugs at most
reasonable costs through bulk purchase and other purchase
procedures;
- operating an efficient inventory and stock control system;
- developing an efficient workable system, where drug
needs of the peripheral institutions can be a gauged
and timely drug supplies ensured.
iv)
Adequate drug distribution through the Government's health
service infrastructure should be ensured. Essential drugs in
adequate quantities and at subsidised rates should be available
at PHCs, and their sub-centres.
v)
Quality essential drugs should be made available from Govern
ment fair-price pharmacy shops. These could be handed over
to PHCs and sub-centres.
vi)
Education and relevant material on good pharmacy management
as produced by WHO should be made available to pharmacy
management system.
9:vii)
Trained and qualified pharmacists should dispense drugs.
D. DRUG INFORMATION AND ETHICAL MARKETING
D
Drug Information
i)
It should be the statutory duty of the drug control authorities
to inform health personnel and consumers of the WHO's concept
of essential drugs, India's graded essential drug lists, drug
policies and their rationale regarding banning of drugs. Rational
drug policy as a topic should be included in medical and paramedical education.
Names of the brands banned for manufacture and sales should
be widely publicized in medical journals, magazines, national
newspapers, giving briefly the explanation and rationale of
the ban.
D
2.
Ethical Marketing
All sales promotion material including package inserts, medical
data sheets by the drug units should be screened by a permanent
National Drug Information body, which will be part of the
National Drugs and Therapeutics Authority. This body should
be responsible for screening as well as ensuring availability
of unbiased drug information to the health personnel and
consumers.
Use of audio-visuals for sales promotion on drugs to doctors
in absence of a printed copy (to be kept with the doctor),
of the claims made, should not be allowed.
All drug promotional literature should contain balanced and
verified scientific information about indication, contra-indications
side effects and drug interaction and antidotes.
iv)
Inadequate and inaccurate information in medical promotional
literature or package insert or worse still of the total commi
ssion of the package insert (as is the trend at present) should
be considered a punishable offence.
v)
Seminars, scientific sessions held by drug companies to present
mainly industry sponsored research studies should be closely
monitored and if need, be restricted as it is associated with
presentation usually only'' of favourable results and tend to
create a sense of obligation in the minds of certain medical
personnel towards drug companies for sponsoring .their research.
vi)
Sponsoring of National Conventions of professional medical
and academic societies by drug industry should be discouraged
since consumers have to ultimately indirectly foot the bill
and such sponsorship inevitably introduces bias in favour of
the company and its products. The health ministry should
take up the responsibility for making funds available for such
seminars.
vii)
Advertisement of tonics and food supplements should not
be allowed in the lay-press. OTC sales advertisements making
false or misleading or inaccurate claims should be banned.
Authorities should ensure that adequate consumer caution
is provided to the consumer in regional languages.
viii)
Labelling should be clear. International non-proprietory names
(generic names) should be used. Consumer caution should
be in regional languages.
For food supplements, nutrients, tonics in the consumer caution
in regional languages it should be added that "This is not
a substitute for normal food" and message given pictorially
wherever possible.
D
ix)
"The International Code for Ethical Marketing" as drafted by
the Health Action International should be adopted by India.
3.
Drug Nomenclature
International non-proprietary names should be used for sales, labelling
and prescription writing. This being so because of several advantages:
i)
Generic drug names are used in under-graduate/postgraduate^
medical and pharmaceutical education.
ii)
Generic names are used in medical text books, scientific
medical journals and WHO publications.
iii)
All purchases of medicines from international tenders and
international markets are based on generic names.
iv)
Use of generic names also ensure production, sale and dispensing
of more rational single ingredient drugs.
v)
Generic name assures clarity by giving information of the
class of drug and thus avoiding confusion arising out of many
dissimilar brand names of one drug.
-dis
Drugs of egual quality are usually cheaper when purchased
by their non-proprietary names than when bought using brand
names.
Use of non-proprietary names is ai valuable aid to memory
as health workers have to learn and remember each drug
by one name only.
E. SELF - RELIANCE
Technological self-reliance
In view of the high importance of achieving the goal of selfreliance in the drug sector, it is imperative that all technology
transfer agreements are made in accordance with the United
Nations Council for Trade and Development draft code.
Protective mechanisms should be evolved for process that
are being developed in the national laboratories so that techno
logy being developed indigenously does not get aborted as
it has happened in the recent past in case of processes deve
loped at NCL and Central Drug Research Institute.
E
iii)
While encouraging in house R&D activity through fiscal incentives, mechanism should be evolved that the R&D effort
undertaken by different firms is in accordance with the priority
drug needs of the Indian people.
2.
Encouragement to Indian Sector
Make priority drugs, already produced in the country from
basic stage by the public sector and wholly Indian companies,
a reserved category for which companies holding foreign eguity
more than 26% should not be allowed any fresh license.
Stipulate a strict time limit of five years for all
foreign
companies to start production from basic stage for the existing
already licensed production capacities.
iii)
Ensure implementation of the time limit of two years stipu
lated for foreign companies to undertake production from
the basic stage for fresh license.
-:12s-
E
iv)
No Carry on Business license or production over the licensed
capacity should be allowed for MRTP, FERA and ex-FERA
companies.
v)
Loan licenses being used by the small scale sector units linked
through ownership, financial participation and/or marketing
arrangements should be cancelled.
3.
Reduction of Imports
i)
The canalisation of all imports snould be streamlined. Open
general licence system should be abolished. There should be
raw material pool in each State to ensure proper pricing and
availability of raw materials.
ii)
Import and excise duties should be fixed in such a way that
the landed cost of imported raw materials and bulk drugs
should not be lower than that of indigenous raw materials
and bulk drug production.
i)
Priorities in research should be guided by the health needs
of the people in India. Drugs required in diseases causing
greater mortality, morbidity, serious sequelae should get
priority. Vaccines should get priority over other drugs.
ii)
Even 38 years after the cessation of British Colonial Rule
in India, research on non-allopathic drugs continues to get
step-motherly treatment. Hence research on these drugs should
be encouraged. None of these drugs, however, should be allowed
to be produced on commercial scale unless their efficacy
and safety has been proved through scientific research.
iii)
Research policy on drugs should be reviewed every ten years
to respond to changing pattern of diseases in India.
iv)
All medical research on human beings must be statutorily
required to conform to the 1975 Helsinki (Mark II) Declaration.
All research proposal must be approved by a central authority
before research is started. This should be strictly adhered
to in case of contraceptive research also.
F. RESEARCH AND DEVELOPMENT
The present policy of giving priority to research on hormonal
contraceptives rather than to barrier methods must be reversed.
-:13:-
G. DRUGS LEGISLATION AND ADMINISTRATION
G
1.
Drug legislation should provide for the following:
a system of registration of all medical products (including
traditional medicines)
enforcement of good manufacturing practice
full control of labelling and advertisement
control of prices of finished drugs and therapeutic raw
materials
prescription control of toxic/poisonous and habit forming
drugs
summary trial for violations against the drug policy by
manufacturers and traders in special drug courts
heavy penalties including confiscation of equipments and
properties for the manufacture and/or selling of spurious
and sub-standard drugs.
The legislation should be reviewed, regularly modified and
updated in the interest of the public and they should not
become bottlenecks for implementation of the national drug
policy.
G
f-
2.
National Drug and Therapeutics Authority
i)
The greatest need of the moment is greater public accounta
bility and a greater social control over pharmaceutical industry.
For this, setting up an independent machinery such as a National
Drug and Therapeutics Authority is imperative, which can
scrutinize all the drugs currently marketed in India on an
ongoing basis and be held responsible for the nature of drugs
in the market. This permanent body should have representatives
with medical, pharmacy and management expertise. Repre
sentation being from :
1) drug and health authorities from states
2) Ministry of Chemicals and Fertilzers and Ministry of Finance
3)
medical professional and medical academic bodies
4)
consumer groups and NGOs involved in health work
5)
6)
Trade Unions related to drug industry
chemists and druggists.
The Government should establish National Drug Authorities
(NDA) at the State level also. The Drug Controllers should
be accountable to NDAs.
ii)
The recommendations of the National Drugs and Therapeutics
Authority should be binding on the drug industry.
iii)
Appropriate powers be delegated to Central Drug Controller
and State Drug Control Authorities for the proper implemen
tation of the recommendations of the Drug and Therapeutics
Authority.
iv)
Relationship of NDA with centre and state drug and health
authorities should be clearly defined. Its constitution, function
ing and powers should be aimed at proper implementation
of National Drug Policy. Suitable drug legislation support
should be given to this authority so that its decisions are
not unnecessarily challenged in the court.
v)
Drugs should be dealt with by this NDA rather than by Ministry
of Chemicals and Fertilizers, to give greater emphasis to
the therapeutic relevance rather than industrial profits and
Government's revenue.
H. HUMAN POWER DEVELOPMENT
Not merely medical and pharmacology related manpower development
is required, but also development of drug managers, drug inspectors,
quality control technicians, researchers and scientists willing to
do R and D in areas of grater concern to the health of our people.
The training and development should include training of legal personnel
who will be dealing with Food and Drug Courts.
Exposure and training of policy makers to other dimension of drug
issues as experienced by consumers and health personnel in the
field is also relevant.
Drug control mechanism has to be developed in keeping with the
growth of our drug industry and be proportionate to our drug prod
uction and sales.
THE ALL INDIA DRUG ACTION NETWORK (AIDAN)
COORDINATION COMMITTEE CONSISTS OF
(1)
Arogya Dakshata Mandal, Pune.
(2)
Catholic Hospital Association of India, Delhi.
(3)
Consumer Education & Research Centre, Ahmedabad.
(4)
Consumer Guidance Society of India, Bombay.
(5)
Drug Action Forum West Bengal, Calcutta.
(6)
Delhi Science Forum, Delhi.
(7)
Kerala Sashtra Sahitya Parishad, Kerala.
(8)
Locost, Baroda.
(9)
Lok Vigyan Sangathana, Bombay.
(10)
Medico Friends Circle, Pune.
(11)
Voluntary Health Association of India, Delhi.
************************
AIDAN Coordinator
DR. MIRA SHIVA
c/o VHAI
C-14 Community Centre
S.D.A. New Delhi 110 016.
Allfl Bndfe
Brygi Aetaon M@fw@rk
-A0DAN
OUR DEMANDS
*
*
*
*
*
availability of essential and life saving drugs (i.e. adequate
production and streamlined distribution to peripheral areas).
withdrawal of hazardous end irrational drugs
availability of unbiased drag information to health personnel
and consumers (including updating our National Drug Formu
lary), and provision of therapeutic guidelines as in British
National Formulary; provision for consumer caution in
regional languages for problem drugs
adequate quality control and drug control so that every
fifth drug in the market is not sub-standard as in at present
according to Government's own figures
drug legislation reform to prevent drug companies from
misusing legalistic loopholes against the people.
Printed at JS Bros, A30/1 Naralna Indi. Area Phase-I
New Delhi 110 028
Do Drugs Cost Less in India?
Amit Sen Gupta
A comparative analysis of drug prices in India and other countries
shows that the average cost of older drugs is highest in India, while
newer pharmaceutical products still under patent protection globally, or
recently out of its purview, are cheapest in India.
the Indian market, formulations of these
drugs account for three, and of the top 20
these account for seven, viz, Althrocin,
Septran, Roscillin, Novamox, Mox,
Ampoxin and Voveran. Althrocin, a
formulation of erythromycin, is the top
selling brand with an annual turnover of Rs
4.24 crore. The second group comprises
three newer drugs. Ranitidine, Diclofenac
and Nifedipin, which are still under product
patent outside India or have come off patents
only recently. These drugs too are fairly
representative with formulations based on
two of them, Zinetac containing ranitidine
and Voveran containing diclofenac, being
listed at the sixth and 20th places respectively
among top selling products (ORG Retail
Audit, December 1994).
The retail prices of these drugs have been
compared in the six countries. Where
different brands have varying prices, the
lowest price has been taken for purposes
of comparison. In order to show the
relative position in different countries,
average cost of each basket of drugs
(comprising six drugs in the basket of
older drugs and three drugs in the case of
newer,drugs) has been computed. This
A MAJOR element of the campaign against (now under suspended animation) is the
changing India’s patent laws, in order to only country in this study,not to have a
comply with requirements of the GATT product patent regime as yet (if one discounts
agreement, has focused on the alleged fact the recent amendment which could not by
that drug prices are lower in India than other passed by parliament).
countries. Hence, il has been argued that a
Drugs chosen for analysis fall under two
change in India's patent system would lead groups. The first group comprises of six drugs
to massive increase in the prices of drugs. - amoxycillin, co-trimoxazole, diazepam,
This claim has been voiced both by Indian ■erythromycin, frusemide and propanololindustry associations as well as public interest which have been in the market for a long
groups campaigning against the GATT time and are not under patent protection
agreement.
(process or product) in thecountries analysed.
While a lot of rhetoric has been used by While an analysis based on these six drugs
both sides in this debate, the claims and cannot be termed as exhaustive, they are
counter-claims have not always been based fairly representativeof thedrug; in the Indian
on hard facts. In order to put this debate in market. Of the five top selling products in
(its proper perspective, an analysis of
comparative prices of drugs in different
Table 2: Comparative Costs of Newer Drugs
countries is presented here. The countries
(Patent Protected or Recently Off Patents)
chosen include four developing countries
Cost
in S of 100 Units
Average
Real
Adjusted
from South Asia-India, Bangladesh,
(Tablets/Capsules)
Cost for GPD Per
Cost of
Pakistan and Sri Lanka, and two countries
Basket
Capita
Basket of
from the developed world - Canada and the
Ranitidirle Diclofenac Nifedipin
of Three in Dollars Three Drugs
UK. Thecountries of South Asia werechosen
Drugs
(1991)
(According
Where Cost
to GPD
as they, broadly, are at similar stages of
in India=l
Per Capita)
development and their economics function
under familiar constraints. The two countries India
2.00
3.00
2.00
1.00
1510.00
1.00
with developed market economies are si milar Bangladesh
2.00
20.00
5.00
4.22
1160.00
5.42
14.00
7.00
2.00
3.06
1970.00
■ 2.35
to the extent that both retain strong state Pakistan
9.00
63.00
2.00
8.83
2650.00
5.03
support to health care and have mechanisms Sri Lanka
31.00
30.00
28.00
1.02
13.11
19320.00
to regulate cost of health care including Canada
UK
73.00
16.00
11.00
12.61
16340.00
1.17
those of drugs. India with its liberal process
patenting system as regard to pharmaceuticals Source: Same as in Table 1.
Table 1: Comparative Costs of Older Drugs
(Not Patent Protected)
Average
Cost for
Cost in $ of 100 Units (Tablets/Capsules)
F
Amoxycillin Cotrimoxazole
Diazepam
Erythromycin
Frusemide
Propanolol
Six Drugs
Where Cost
in India= 1*
Real
Adjusted
GDP Per
Cost of
Capita
Basket of
in Dollars Six Drugs
(1991) (According
to GPD Per
**
Capita)
3.00
India
12.00
1.00
1.00
1510.00
1.00
9.00
3.00
3.00
.
—
Bangladesh
1.00 .
0.77
1.00
6.00
10.00
1.00
1160.00
‘ 3.00
1.00 !
5.00
Pakistan
5.00 ‘
0.60
0.65
1970.00
0.50
3.00
3.00
■ 0.34
Sri Lanka
0.14
5.00
'
0.60
0.60
2650.00
4.00
1.00
0.19
—
6.00
6.00
0.81
Canada
8.00
0.50
0.50
19320.00
0.06
—
0.82
7.00
6.00
16340.00
0.08
UK
, 5.00
1.00
* Calculated by taking cost of each drug in India as 1 unit and computing the relative cost in other countries. Then the average of thf basket of drugs has
been taken. Where data for all drugs not available, average computed on the basis of number of drugs on which data is available.
*• Calculated by multiplying average cost with the ratio of GDP per capita in India, with the corresponding figure for each country. This gives a rough
measure of the financial impact of buying drugs in each country.
Source: K Balasubramaniam, ‘Retail Drug Prices in Asia-Pacific Region’, HAI News, December 1995.
Econom1-’ at '1 r .ittcal Weekly
January 27, 1996
195
computation was done by taking the price
of each drug as one unit in the case of
India. Based on this the relative cost of
each of the drugs in the countries studied
have then been calculated, and a mean
value for each basket calculated from this.
To show' the real impact of drug prices the
relative cost and mean cost adjusted
against the GDP per capita has also been
shown.
The analysis is shown in Tables 1 and 2.
We see from Table 1 that the average cost
of older drugs is the highest in India. The
cost is three times that in Sri Lanka and
even higher than in UK and Canada.
Adjusted against GDP per capita, cost of
these drugs works out to be five times that
in Sri Lanka and 12 to 16 times that in UK
and Canada. The position is the complete
reverse in the case of newer (patent
protected) drugs. Table 2 shows that in the
case of these drugs, prices are lowest in
India. These drugs are three to 13 times
more expensive in the other countries
studied. Even when adjusted against GDP
per capita the costs of these drugs work
out to be the cheapest in India.
This interesting outcome lays bare the
half-truths and lies resorted to by the two
contending industry associations in the
pharmaceutical sector in India, the Indian
Drug Manufacturers Association (1DMA)
(representing Indian companies) and the
Organisation of Pharmaceutical Producers
of India (OPPI) (representing multinational
companies). The IDMA has consistently
argued that drug prices are the lowest in
India and a change in patent laws would
reverse this position. The above analysis
clearly shows that drug prices are lower in
India only in the case of patent protected
drugs. We find from our study that in the .
case of other drugs, prices are higher in
India than even developed industrialised
countries. Given the fact that drugs in the ■
Indian market, which are under product '
patents globally, account for only 10-12
per cent of total pharmaceutical safes, this
means that by and large Indian drugs are
costlier.
This is indeed a strange situation as
logically India should have an edge over
almost any country in the world in this
respect. Unlike Pakistan, Bangladesh and |
Sri Lanka, India has the indigenous
capability to manufacture most drugs.
Further economies of scale should favour .
Indian manufacturers in comparison to these
south Asian countries, given the larger size ■
of the Indian market. Compared to UK and
Canada, Indian manufacturers enjoy the
advantage of lower infrastructural and
labour costs. A conclusion one can draw
is that the industry in India is cither •
unwilling or incapable of passing on the ,
196
results of these gratuitous circumstances to
the consumer. In fact, to the contrary,
companies in India (both Indian and MNC)
have received a further bonus in 1995 in the
form of the new Drug Price Control Order,
where price control mechanisms have been
further relaxed by drastically reducing the
Span of control and by increasing the
profitability allowed in drugs still under
price control. If the industry still wishes
to claim that the new DPCO is fair, it would
lay itself open to the charge that Indian
industry is not globally competitive. This
is a factor worth exploring and would point
to a high degree of technology obsolescence
in the industry.
Table 2 exposes the claims of the OPPI,
which argues that a change in Indian patent
laws will not affect drug prices significantly.
The OPPI has consistently claimed that drug
prices for patent protected drugs will be kept
in check by India’s drug price control system.
What our study shows is that in fact India’s
price control system has been incapable of
pegging drug prices even at the level of
prices in some developed countries. The
comparatively low prices of newer drugs
have been in spite of the lax control
mechanisms, solely as a result of India's
liberal patent laws. Scrapping of the 1970
Indian Patent Act would do away with
whatever little relief Indian consumers
could avail.
In conclusion, the above study raises some
important fundamental issues. It shows that
India's drug pricing mechanism has proved
to be ineffecti ve in keepi ng down drug prices.
The benefits of the advantage that the Indian
pharmaceutical industry enjoys overall other
third world nations, in termsof the avai 1 abi 1 ity
of indigenous technology and a large
domestic market, have not been passed on
to the consumers. The second point of
concern is the tacit support that the industry
has received, regarding its claim that drug
prices in Indiaarecomparatively lower, from
some public interest groups opposing a
change in India’s patent laws. While the
tactical necessity of aligning with Indian
industry associations like the IDMA is clear,
the reasons for adopting their rhetoric in full
measure are obscure. It probably points to
the need forexercising proper vigilance when
such short-term alliances are worked out,
lest the charge of co-option be laid at the
door of public interest bodies. It also points
to the need for such bodies to critically
examine arguments put forwar^fc the
industry, before putting the stamp ofapprova
in joint platforms.
SCIENCE AND EMPIRE : Essays in Indian Context (1700-1947)
Edited by Deepak Kumar
This book is a collection of fourteen well-researched papers focussing
attention on how.science and technology were used for imperial purposes,
the close links between the two, and what response it elicited from the Indians
These papers help not only in placing scientific and technological works ir
British India within colonial parameters but also in understanding the mecha
nism of imperialism itself.
ISBN 81-85150-19-2
Rs. 30
SCIENCE, TECHNOLOGY AND COLONISATION
£
An Indian Experience (1757-1857)
Satpal Sangwan
What Place science had in the process of colonisation? What was the type
scientific development that India experienced under colonial relations?
what way the local people responded to the new descriptions of science ai
technology? These questions call for a closer analysis of the nexus tb
developed between science and imperialism. This book studies scienti
development in India during the first century of colonial rule in this perspectk
ISBN 81-85150-11-9
Rs. 3
A ANAMIKA PUBLISHERS & DISTRIBUTOR
/
W
29A, Pocket D, Deep Enclave, Ashok Vihar III, Delhi 11005
Economic and Political Weekly
January 27
Analysis of Drug Prices, 1980 to 1995
Wishvas Rane
Since the drug policy of1986, many changes have been introduced liberalising the environmentfor drug manufacturing
and marketing. These changes have apparently come about as a response to the drug industry's plea that controls
in India were too stringent and that without controls prices would stabilise and not rise. Yet, a categorywise analysis
of prices of drugs over a period of 15 years shows that there is a general rising trend in prices, especially of essential
and life-saving drugs.
IT is difficult to work out an ideal drug price
index, because of the many variations in
diseases and their treatments. It is also
difficult because of different prices of
different brands of the same medicine. But
there is need to find out the real rise (or fall)
in drug prices.
The drug industry often claimed that drug
prices have not increased when the prices
of other consumables have risen. This claim
is normally accepted by the government
which then pats its own back for the success
of its Drug Price Control Orders (DPCOs)
to contain drug prices. The Consumer Price
Index for urban industrial workers increased
by 273 per cent during 1980 to 1995 (April).
This rapid rise was due to the unprecedented
rise in food prices during this period. It
would therefore be apt to compare drug
prices with those of an industrial consumer
good like clothing and footwear The price
index for this group has increased by 170
per cent during this period.
When we consider drug prices, we should
certainly consider the Hathi Committee
comments on drug prices. It says “In
appreciation of the fact that ill-health has
major socio-economic implications, the
committee feels that in a welfare state,
availability of prophylactics and curatives
should receive the highest priority on par
with food and shelter. Production and
distribution of drugs should, therefore,
constitute an important social responsibility
of the state. The committee is of the opinion
that trade aspects of this vital industry should
be divorced from the ordinary accepted
principles of trade for profit”.
The Drug Policy (DP) of 1978 and the
DPCO 1979, were based, albeit partially, on
recommendations of the Hathi Committee.
Forthefirsttime,comprehensive price control
was introduced in the drug industry (though
some price control measures had been in
force since 1970). In the aftermath of the
1978 DP. the organised Indian private sector
created a solid base for itself in the drug
industry. Having done so, itjoined thcMNCs
in the campaign for reversal of the 1978 DP.
By the early 1980s. the industry, led by
MNCs, started making belligerent noises for
reversal of the 1978 DP and for decreased
controls. The industry argued that drug
production was becoming unprofitable and
Economic and Political Weekly
even proceeded to deliberately reduce
production of essential drugs.
An RBI analysis of the working of FERA
and othercompanics reveals that theprogress
of the industry in the 1980s has been quite
impressive. The increase in turnover in
relation to the investment in fixed assets, has
been more than proportionate to the
additional capital outlay on modernisation
and expansion, and dividends on enlarged
capital could be paid liberally out of profits.
A large portion of profits after tax was also
retained in the business. The industry had
not had any difficulty in finding the requisite
resources for modernisation {.Kothari 'sYear
Book. 1987, p A 181).
This shows that the industry was making
more than sufficient profits and there was
no reason for asking for higher prices.
From these reports it may be concluded
that thedrug industry as a whole, both Indian
and MNCs performed well in financial terms,
and the campaign of the industry about less
profitability due to DPCOs was misleading.
In March 1985, the central government
delicensed 94 bulk drugs making the policy
of reservation of drug licences almost
infructuous. The 1986 policy, in one sweep,
reduced the span of price control from 347
bulk drugs to 166 drugs. It decreased the
number of categories under price control to
two and increased the mark-ups on these to
75 and 100 per cent.
In September 1994, came modifications
to DP 1986, when different maximum
allowable post-manufacturing expenses
(M APE) were done away with and a uniform
M APEof 100 percent in all cases introduced
for all drugs under price control. The number
of price controlled drugs was brought down
from 142 to 73 and greater profitability (up
to 4 per cent) allowed for bulk drug
manufacturing. By offering such a liberal
drug policy, the government has conveniently
forgotten that even developed market
economies treat the drug industry differently
from the consumer goods industry. Price
and production controls are enforced in all
developed countries.
In order to prove that the drug prices have
increased substantially, an attempt is made
here to compare the prices of drugs in June
1980 to those in April 1995. using data from
MIMS India, Vol 1, No 1 (June 1980) and
MIMS India, Vol 15, No 4 (April 1995).
August 24-31,' 1996
Drug prices of all the products and packs,
showing rise or reduction in prices have been
considered and the prices of a single unit of
such products are totalled. Some 778 product
packs have been compared. This is roughly
55 per cent of all the products of June 1990
as listed in MIMS. For want of space only
product packs showing a rise of over 100
per cent have been listed with respective per
cent price rise. There were 118 product packs
showing a price rise of less than 50 per cent,
145 showing a price rise between 50 to 100
per cent and 50 showing a decline in prices.
From Table 1 we find that there has been
an overall price rise of 196.58 per cent,
which means that the drug prices have nearly
trebled during 1980 to 1995. The maximum
rise of 336 per cent is seen in drugs meant
for treatment of cancer. The radical cure for
gout is allopurinol and the price of Zyloric
has gone down by 79 per cent. All these anti
cancer products are imported and the govern
ment should make an effort to see that these
emergency essential drugs are imported and
marketed at reasonable cost to consumers.
This is followed by anti-allergic drugs
showing a 259 per cent rise. The cheapest
chlorpheniramine maleate is just not
available, though over 20 brands use it in
their combinations. The price of the popular
brand Avil (costing total Rs 2.92 for 1 tablet
thrice a day for seven days) has marginally
increased, but the drug has been rapidly
replaced by cost! ier brands such as astcmisole
(Astclong 1 week treatment Rs 35.80),
terfenadine (Histerf - Rs 45.95), loratadine
(Lorfast - Rs 27.65),- clemastine (Tavist Rs 61.10), and cetrizine (Zirtin-Rs 17.15).
Drugs acting on the respiratory system
have registered a rise of 258.45 per cent and
the maximum rise is shown by cough
mixtures. Drugs acting on the alimentary
system show a rise of 243 per cent, and
strangely enough, inessentials like enzymes
(334 per cent), anti-diarrhoeals (476 per
cent) and laxatives (300 per cent) rise is
seen. This is followed by other inessential
nutritional products which show a 236 per
cent rise. Food products show a rise of 654
per cent, followed by minerals with 265 per
cent, anti-obesity drugs with 193 per cent,
vitamins with 186 per cent and tonics with
185 percent rise. Hormones, most of which
are needed by women, show a rise of 221
2331
per cent. Thyroid treatment .is taken for .a ' (296 per. cent). Now most of the anti-,. ?per cent), Merckenzyme (354 per cent)”,
long time and these products have gone up diarrhoeals have been banned and thus ' Molzyme (372 per cent), Unienzyme (397
by 532 per cent; gonadol hormones'by 409 Streptomagna and Thalazole have been per cent) and many others. Then comes the
per cent. Among the drugs for the musculo withdrawn. Hepatobiliary drugs follow with laxative group with a total rise of 300 per
skeletal system, inessentials like rubefacients a total rise of 355 per cent. Hepasulfol cent. This is topped by Fso-gel with 593 per
(balms) and muscle relaxants ;haye gone.up.; containing trjthipparamethoxyphelylprppene . cent, Dulcolax with 451 per cent i’ts
(a passing reference is made to it jn suppository with 446 per.cent, Pursenid - ..
by 280 per cent.
Only antibiotics have shown a very modest Martindale, as a drug increasing saliva and IN by 351 per cent, Cremafin by 311 per
rise of 64 percent, but few ofthese antibiotics bile and hot included in British National cent and others. In the antacids most of the
are very widely used today. The prices of ’ Farmulae) shows a rise of 602 per cent, ’ liquid preparations show increased prices
chloramphenicol products (Chloromycetin, closely followed by Festal (an enzyme and the maximum rise is shown by Disogel.
Paraxin, etc) have gone up, but even this preparation containing bile salt) by 400 per Among gastro intestinal sedatives, Dimol
antibiotic has been replaced by ciprofloxacin cent. Festal is now being sold with a changed and Epidosin have shown the maximum rise.
and others. The newer-antibiotics are composition - the bile salt has been dropped In this group the inessential drug
introduced with higher prices and these are and the product named .Festal-N with the combinations for hepato-biliary problems
same price.' Enzymes show a rise of 330 per and enzymes show a big rise. The prices of
extensively and vigorously promoted.
From Table 2 we find that the maximum cent. Panzynorm, an enzyme combination commonly needed laxatives have risen by
rise of 476 per . cent is >'shown by anti- . containing bile and stomach extract, has four times: Within a year (March 1995 to
diarrhoeals, wherein a substantial rise of 768 registered a rise of 1112 per cent. Bilamide March 1996) prices have further increased
per cent is seen fonSalazbpyrine which is is a brand of nicotinamide and indicated for for Aludrox and Gelucil-MPS antacids, p
probably the only available remedy for anorexia, flatulence, dyspepsia and Buscopari and Serebantine antispasmodics,
ulcerative colitis. This is closely followed constipation, shows a rise of 552 per cent. Dulcolax suppository and I-so-Gel laxatives,
by Thalazole (574 per cent) and Walamycin This is then followed,by Bestozyme (416 Salazopyrine anti-diarrhoeal, Sorbiline and
Table Ii'Price Rise -Systemwis'e '1 '
Pharmacological 4 ■■ •
Indexj.y. :/ii\
Antacids • *.
■ '■
’*
.GI Antispasmodics
i <:
Laxatives _•; ■• • ■
J ;7 j
Colon and rectum
Anti-diarrhoeals
Hepato-Billiary
Enzymes • • '•■■■■
Total alimentary system
Cardiac disorders
Anti-anginals , . j
-, u .
Peripheral vasodilators
Anti-hypertensives' ■ ‘
Anti-inigraine
‘I
Anti-coagulants
Haemostatics
.
Total Cadiovascular system
Analgesics
Hypnotics
‘
Sedatives, tranquilliser
Anti-depressants
Anti-emetics
Anti-conyulsants
. .. .
Rigidity controllers
Total Central Nervous System
■ Anti-inflammatory-NSAID
Muscle relaxants'.
•,’>/. :
Rubefacients-Balms
.
•Total musculo skeletal system
Gonadal Hormones'
Oral contraceptives
Corticosteroids
Hypoglycemics
• •; L
.Thyroid and Antithyroid
Total Hormones .
Diuretics
Urinary anti-infectives
Vaginal and urethral
Uterus
• . ../fi: ‘
,Spennicidals
Total geuito-urinary system .
Respiratory stimulants
• Broncho-spasm relaxants •
Expectorants ■
,■d■
Total respiratory system ;.
Antibiotics . .. . c .,
:2332
Price
. Per Cent’ ’Pharmacological ’
Price ,
■ perCent
1S»8O • < . 1995 , ;
-Rise
-.hI?80 .
.. (1995.r
.Rise,-. ■Index-.
’143.11
148.95 - . -4.08M
84.50 . Sulphonamides :
■94.51 :
174.37
Anti-tubefculours
340.12 ■
341.33
: 0.36”
91.70
67.98
130.32
184.50 .. 180.00
reduced
155.40
299.90 ;.. .. Antileprotics
..38.86
Antifungals
20.94
84.33
302.72
71.67
17.15
9.99
37.42 ‘
Antiamoebics . .
63.90
70.76
475.77
55.80
321.28
■ 5.71
16.68
192.12
122.37
26.88
355.25 ‘ ? Antimalarials
Antihelmintic
29.07
38.59
: 32.75
95.72 • 415.30 ■ 333.87
193.11
325.82
Vaccines • •. Xi-Tr • •
' 45.35 .
242.8
64.21
119 20 • Total infection and ^infestation
188.62
86.05
76.48
214 *61
180.61
Tonics
\. ?
204.62 .-. 173.63
74.78
Iron preparations' :
345.64
. 134.17
’157.61
76.30
72.35
127.62
Minerals and vitamins
234.05 ' ■ 854.33
265.02
177.23
125.25
78.68
’. 521.11.. . 1490108
185.94;
• 57.00 . . Vitamins'
12.86
20.19
Antiobesity
. 14.35
. 42.05.
193.03
■: .5.15
,80.25 : ■ ■1458.25
Food products
82.42 . ; 621.74
654.36
34.07
94.35
17693
• Total nutrition
’ 235.83 ’
145.34
Nose ■■ .• ’■ •
45.80 i '■ 145.24' ‘ ’217.12:
103.81
'67.70
137.98
.Throat, mouth
■ 9.75
17.08
75.18
90.58 • 268.51
' 24.58
;r. j: 47.09- .
87.29 . ••',85.37
193.66
203.92 . Ear :- ; : . . : b'63.72
-.<-<> • • In:’. >1 : ■.
143.19 .
218.16 . . Total ear, nose, throat
14.59 ' . 46.42
147.57 ' .107.52
198.34
121.19
63.66 .. Anti-infective . . . . . . . . .71.11
11.04
24.86
125.18 '
99.24
397.60. . 300.64 •.-r- Anti-inflanimatdry;
■ 29.58’
97.56
'229.82 .
344.50 , - 334.81 ' ""Glaucoma
79.23
• tilt ,
’’ 12.00 ■
14.47
-20.58
■ 199.64 . •'•■ Mydriatics and cycloplegics •
■-38.54 . 1.121.30 . 214.74
66.38 . i ’; ’ .-Diagnostic-and miscellaneous .
43.79
26.32
' 150.05
33.42
: 127.29.. i 280.88 . 7, Totai ophthal mic ’ ‘;
.
,59.23 . 288.07 • .Antiallergic
, . 258.5
15.26
207.07 , ,'.7; Total allergic manifestation .. .196'50
258.5W
'408.56 . /Soothing and protective ..j,
151.99.4 772.96
11.32 '
85.90
658.83 :,14.40
195.08 .'keratolytic and cleansers
'
4.88
26 49
109.27
312.50 .
235.50
441.97 ■ 97.67 ? Topical. NSAID, antipruritic
4.08
13.91
240.93
■7
148.63
182.63 ■/.
73.99 Antifungal an’tiinfective
46.07
115.45 :
150.60
531.81
. 169.64
26.85
Antiinfectives
•
u: 67.83
168.72
148.74 .
. ‘ 220.67 . . ’ Topical steroid
32.44
79.49
129.62 •
21.54
66.21
■ 80.47
.Miscellaneous
92.76.-. 256,06 .,,.176.37
193.41
171.15
71.33
Total Skjn.
194.96
'166.60
202,36
55.10
Carcinochem therapeutic .
738.37
3756.76 : 408.79
•48.85
109.57
■ . 23.31
Immunosuppressants
•223.41
271.67
878.60
33.47, . .. 432.11
,
6-29 ■
57.88 . . '12.04. * (educed
135.24 • ‘ Anti-gout
.
■ '29.20 '
35l‘.34
131.79
' . "17.50
32 87
' 87.83 ■lf Poisoning’
Total metabolism
335.61 .
229.71 • ■ 202.17
■ 76.02'
. . .•
692.41 •> • -300.47 ' hv Grand Total ,i
• • 172.90 •
258.45 . All Systems . . .
775 formulations
. , '
1419.11
100.51
'.. 707.74 .
. '.196.58 . ■
.Economic and Political Weekly
August 24-31,' 1996
Table 2: Alimentary System ■
Stimuliv’for hepato-biliary systems and
Bestozyme, Digiplex, Dispeptal enzymes.
.Table 3 shows comparative prices ofdrugs
used for heart diseases. The total rise is 135
per cent, but most of these drugs arc not
widely used and recentlyiintroduced newer,
drugs for higher prices are being promoted.
In. .this
rise is1 shown
.. group^ ..the’maximum
....-----by the anti-coagulants that are nee'ded by
•
• '
cardiac patients for a life time, Acitrom,
the
only' available product, shows price rise of
2359 per cent for a 4 mg tablet and 1417
per cent for a 1 frig tablet. In the following
year prices have been reduced- (1 mgx 10
tab @ Rs 18.15 and 4 mg x 10 tab @ Rs40.60). But even if we'compare the reduced
prices to the prevailing prices in 1980, we
find the rise is 1152 per cent for a 1 mg tablel
Antacids
and 21 19 per cent for 4 mg'tablet. Uniwarfm’,
Alucinol
0.96 1.98 108.42
shows a modest rise of608 per cent.'Among '
Aludrox
350 ml 7.35 17.08 132.38
Disogel
the drugs for cardiac disorders, Lanoxin-:
175 ml 5.40 18.55 243.52
. Gelusil
170 ml 6.10 13.29 .' 117.87
paediatric shows a rise of 579 per cent,
Mucaine
175 ml 9.06 18.77 100.55
closely followed by Natcardine - 251 per
Polycrol fort 175 ml
13.04 131.21
cent, 'and .-323 per cent-for Mephentin'
• Solacid
1.33 2.72 104.51
injectible.-Among anti-anginal drugs’
Rise less than 50 (9)
Angised shows a rise of 548 percent and
Rise 51 ro 100
Neocar'249 per cent. .In the peripheral
per cent (8)
T
able
3:
C
ardiovascular
S
ystem
....
.
vasodilators, Arlidin shows a rise of 415 per
Gastro-intestinal sedatives
Buscopan
10T
8.47 147.66 Product
cent
and Nicinal 240 per cent. The, role of
.Pack
Price - Per Cent
1 ml
3.41 136.81
' . '1980 1995 Rise'. these vasodilators for cardiac conditions isb
Colimex
10 ml 2.66 10.00 275.94
doubtful. Among theanti-hypertensives. the
RsPs RsPs
Dimol
10T 1.16 5.00 331,03
prices of methyldopa (Emdopa-- 136 per
Epidosin
. 20T 4.16 1'9.06 358.17 Cardiac disorders
Pro-banthine
3.90
139.74 Betabio forte'.. 12T 4.14 11.22 171.01 . cent, Meldopa- 396 per cent) have gone up.'
Cardioxin
Sere-ban thine
20.T 1.83 3.93 114.75 Among haemostatics, Gynac CVP has a very.
4.65 12.21 162.58
Ciplar 10 mg
Spasmo10 T 2.00 4.3f ; ’,115'50, limited role to stop bleeding, but it shows
20 mg . ; . TOT 4.10 10.56 1'57.56. a rise of 154 per cent. .Premarine is useful
. proxyvon
4.55 9.56
Indcral 10 mg
; Reduced (1)
3.54
10T i.73 4.91.183.82 in uterine bleeding (dysfunctional) and prices
40 mg
Less than 50 j
16 T .4.08 :11.80 189.22 have gone 'up
up by 185 per cent.iThe'.priccs
80 mg
222.87. of Persantin, Duvadilan, Ismelin andNepresol
percent(3) •HOT «.00 15.83
------------Lanoxin
50-100 per cent (1)
10T 0.91 2.02 121.98 have reduced. Ciplar, Inderalwanti- '
Paed elixir
30 ml 2.69 18^27 579.18
223 7 8- hypertensives, Adelphae essidex,-Emdopa
Injection
Agarol
170 ml. 8.74 33.36 ,.281.69,
20 T :6.02 25.50 323.50 have: further.increased over pricesiduring
Cellubril
30 C : 9.06 22.00.142,83 Mephentinc
1995-9.6, whereas Inderal 10 mg, Lanoxin
•1ml ;1.8I 5.30 192.82
Cremafin
210 ml 5.62 23.10 311.01
Natcardine”' ■20T 10.63
2_.'_ • 37.32 251.08 injcctible, Angised, Acitrom have shown a
DulcolaxRise
less
than
50
per
cent,(1)
1
suppository
decrease in prices. The price of Lanoxin
y
10T 1.18 6.50 450.85
10mgx5 4.50 24.55 445.55 Rise 50-100 per cent (1) ,
tablets has increased during 1995 to 1996
(2) *
• '*
;,
Evavuol
75 G 6.21 24.43 293.40 “Reduced
‘
to Rs 4.16 registering a rise of357 per cent.
,
I-So-Gel
52 G 2.25 15.60 593.32 Antiangihal and \ujsqdila'tores
The
highly priced new entrants like Corive'rsyl
I00T 5.08 :32.90. 547.64.
Pursenid-IN
10T 1.30
_____________
’; 5.86 350.77 Angised
(10
tabs
@-Rs
-220.60),
Inocor
(20
ml
Ildamen
.
2ml
1.4
1
3.18
125.53
Drugs acting on colon and rectum
.Neocar
..---100 T 28.55. <99.55 248.69 @ Rs 933.00),iNorpace (100 mg x 10.tabs
less than 100 •
; •/
@ Rs 37.74), Flavedon (10 tab @ :98.93)
Peritrate SA
10 T'’2.87 8.20 185.71
percent (2)
Rise less than 50 per cent (3). ?
Anti-diarrhoeal .
;• ’
have shown varying rises during !995-96.
■
Salazopyrin 0.5g 50T -i.29.39..255,00. 767.64 Rise 50-100 per cent (3)
Table 4rgives-the comparative.prices of
Streptomagna I0T 3.17' "-8.72n
175.08 Reduced (1)
the drugs acting bn central nervous system;
Thalazole;
I0T 1.32 8.90 '574.24 Peripheral vasodilators, ' •
25 T ‘6/11 31.50 415.55 i -e. pain-killers.'fever-reducers, hypnotics;
Walamycin . 30 m! 4.29 17.00 296.27 Arlidin
Nicinal
., -10- T- f 2.07 7/04 240.10 sedatives/.anti-depressants.'anti-nauseants;
Less than 50 , , ■ 'r
anti-convulsants, etc.Tn this groupthe total
Reduced (1) '•
\ ' 3.46 2.16
per cent (.. ' ’ (3)
prices have trebled. Thc.maximum riseidf
. • Rise less than 50 per cent (I)
50-100 Per cent (20 ’
Rise 50-100
Hepato-biliary .
335 per cent is shown for drugs used.dn
per cent (2)
60.71 86.92
Festal
r --JOT 5.00 25.00 400.00
Parkirisoism, closely followed by ti 301 .'per
*
602.25 Antihypertensives^
Hepasulfol
60 T 8.43 .'59.20
cent rise for anti-convulsantsjused for
.... 19.55 173.81 Brinerdine
' JOT
,fs'r 8.34 -32.09'284.77 epilepsy,’269 pier cent for hypnotics, arid so
Sorbiline
J00 ml . 7.14
• 10 T . 6.48 15129 135.96
Stimuliv . 100 ml11 ’6.31 '.18'62 ■ 195.09 : Dopagyt :■'
; Emdopa .-.-j '. ( TOT :6J5 ,30,49 395.77 on: Among analgesics', the maximufn riseof
Enzyme ■. ■
181 per cent is' shown by Micropyrin, a
10T.6.37. 24.14.278.96
Bestozyme
30 T 4.62 23.85 416123 ■ Meldopa
brand of aspirin, Veganin another aspirin
12.26 8.84
100 ml 5.68 12,61 122.01 . Reduced (3)
combination shows a rise of 178-per cent.
Bilamide .
30 T 10.92 71117; 551.74 Rise less than 50 per cent.(3). ‘ >
Digiplex . 100ml "6.35 2L69 241.57 Rise 50-100
Injection Fdrtwin and Sosegon show a rise
39.08 60.38
per cent (3)
Dispeptal
.10 T 4.50 18.73 316.22
of 174 and 163 percent, respectively. Calpol
Lipizyme
210 nil '7.38 26.00 252.30 Migraine
and Crocin, the popular .brands of
Merckenzyme TOT 10.54 47.85 353.98 ' Rise *less than 50,per ceni.(i j
paracetamol show a rise of o9er 100 percent.
t Molzyme
30T 6.14 29.00- 372.31 : Rise 50-100 • •'V ■'
In this group, Calpdl tablets. Cofamolsyrup,
Normozymc? 25T 4.52 • 9.33 ‘I06f42
per .cent (2) .
.-.12.86 ^20.19.
Crocinsyrup, Fortwin injection, Micropyrin, •.;
Panzynorm . 25 ;T 7.24 87.72 1111.60 Anii-'cpgulants
Anti-cpgulants.■
16.T
1.45
.
22
001417.24
SNoyalgininjection.andpyrigesichaveshowh
Uniemzyme_j.x-L„25
‘
----~~
T "4.53
22.50:396.69
Acitrom I mg
Vitazyme
10T 1.83 , 45.602359.02 a furtherJrise1duringnl995-96.-Hypriotics
110 ml 4.60 14.79 221.52 ’4
Rise less than
Uniwarfin . •25T, 1.87 .,13.25.,608.56 show
showanoverallriseof269pcfcent.TriclorJ'l
an overall riseof 269 per cent.Tricloryi
j 50 percent (1)
;
; Haemostatics'
shows a maximum rise of 502 per c$nt and
Rise 50-100 per cent (2)
Gyna CVP > > i 25 T' 1,9.22 23.43 154.12 it further increases to.Rs'38 (frorii.Rs 33 in '
Total-alimentary j
•Premarin ;t ■ 20 mg. 24.85 70.92 185.39 1995), during 1995-96.iGardcnal, the drug
system^
390.44 1336.19 242^23 Total .)>
368.94 886.88,.140.39 of choice m epilepsy,' has not been available
Product
Pack
Prices Per Cent
1980 1995 Rise
Rs Ps Rs Ps
Econbriiic-and Political Weekly
August 24-31, 1996
.
.
'
.'2333
Table 5: Musculo Skeletal Disorder , ’ .
Table 4: Central Nervous System
through retail chemists (and the drug
controller cannot do anything about its non Product
Pack
Prices PerCent
Pack
Prices ' Per Cent Product '
availability) and has shown a rise of 177 per
'
1980 1995 Rise
1980 1995
Rise
. Rs Ps Rs Ps
Rs Ps Rs Ps i . . ?
cent. Among sedatives and tranquillisers,
the prices of brands of diazepam (Calmod Analgesics
NSAIDS
824 per cent. Calmpose 471 per cent and Calpol
10T 1.45 3.10 113.79
Idicin
p.. . • 10T r. 2.24 4.70 109-82
Suganril , . 10T 2.52 6.39 153.57.
. 60 ml 4.83 .11.75 143.27
Valium 582 percent) increased, but dropped Cofamol
5.62
162.62
Zolandin 200mg I0T 3.24 8-38 158.64
6T
2.14
Corbutyl
marginally during 1995-96. Larpose is a
Reduced (2)
7.83 6.08
syrup 60 ml 4.22 8.50 101.42
brand of lorazepam which is a short act Crocin
Rise 50-100
1 ml 2.45 6.72 174.29
Fortwin
ing benzodiazepam that has shown a rise Mazetol 200 mg 10 T 8.06 17.96 122.83
10.49 18.24
per cent (4)
Muscle
relaxants
of 344 per cent. Librium is a brand of Micropyrin
TOT 0.78 i> 2.20 182.05
Carisoma
10T 40.6 24.85 512.07
10 T 2.28 5.33 133.87
chlordiazepoxide which is a benzodiazepam Novalgin
Carisoma
2 ml 1.33 2.99 124.81
with general properties similar to diazepam,
compound
10T 4.00 15.95 298.75
1.45 2.97 104.83
Pyrigesic
has shown a rise of 408 per cent. Majeptil
Epidosin
1 ml 1.00 5.27 427.00
60 ml 3.35 8.51 154.03
. 20 T 9.94 38.10 283.30
is abrand of thioproperazine which is similar Sosegon
1 ml 2.67 7.03 163.30 Parafon
Robinax .
10 ml 3.45 11.55 243.78
2 ml 1.42 2.97 109.15
to chlorpromazine (a very cheap drug) but Ullragin
, 8T 5.20 18.04 246.92
0.90 . 2.50 177.78
is highly priced with a rise of 1070 per cent. Veganin
Robinaxol
10T 5.77 13.53 134.49
Rise
less
than
50
per
cent
(
I
)
This group shows a total rise of204 per cent, Rise 50-100 per cent (9)
Rubefacients
Algipan
40 g 5.06 25.50 403.95
whereas the anti-depressants show a rise of Hypnotics
Medicreme
30
g 4.92 17.47 255.08
218 per cent. Anti-emetics totally show a Gardenal 30 mg 500 T 11.12 30.82 177.16, Relaxyl .
'30 g 5.28 16.25 207.77
modest rise of 64 per cent, but popular Hypnotex 5 mg .10 T 2.32 7.31 215.09 Total
75.00 230.30 207.07
’ • 30 mg 25.T ' .1.13 3.03 168.14
brands like Stemelil (708 percent) and Siquil Luminal
10 T 2.50 7.30 192.00
5 mg
. Table 6: Hormone
•
injectible (414 per cent) are on the higher Nitravet
349.26
9.12
____
2.03
Sedyn
side. Anti-convulsants are mainly used by . Trichloryl
Pack
Prices * Per Cent
50 ml 5.48- 33.00 502.19 Product •
1980 1995 -Rise
epileptics, for whom phenobarbitone is a Sedatives and tranquillisers
10 T 2^07 7.00 238.16 Gonadal Hormones
good cheap drug, but is not made available . Anaterisol
1 ml 4.35 29.00 566.67
by retail chemists and instead cdstlicr newer
Aquaviron
1ml
1.48
.’ 14.75 896.62
10 T 0.72 6.65 823.61
Duphaston
JOT . 19.80 113.20 471.72
drugs are used which have registered a big Calmod
Calmpose 5 mg I0.T 1.40 8.00 471.43
Gestanin
20T
13.01
53.80 313.53^
rise, viz, Epsolin-590 per cent, Eptoin-402
60 ml 5.13 16.90 229.43
Lynora! 0.01
20T 1.05 18.70 168O.sB
2 ml ; 1.34 7.00 422.39
per cent, Garoin-311 per cent and Mysoline
20T 1.94 23.60 1116.4”
0.05
344.44
6.00
“
“
1.35
Larpose
I
mg
552 per cent, with a total rise of 301 percent.
Mixogen
20T 3.55 27.60 677.46
1.30 407.69.
6.60 ----10 mg 10T
1ml 9.23 31.30 239.11
The total rise in the rigidity and tremor Librium
1.73 20.24 1069.94 Orgalutin
Majeptil 5 mg
100T 23.87 220.00 821.66
control drugs is nearly 335 per cent, nearly Placidox 2
I0T 1.26 4.23 235.71
10T 5.53 26.30 ,375-59
Orgamctril
•
231.72
4*6 times. These drugs are used by patients
10T 2.08 6.90
5
Primolut N
I0T 6.66J 32.00 340.48
10.69 193.68
10T
of Parkinsonism and these have to be used
Proluton Depot 250mg 7.60 32.36 325.79
- 10
10T 2.50 5.00 200.00
500mg . 14.00 56.55 303.93
for a long timei Kemadrinc has increased by Scrcnase 0.25
304.11
10T 1.46 5.90
1ml 9.23 30.00 225.03
Sustanon 100
505 per cent and Pacitane by .611 per cent. Serepax
Valium 2 mg
10 T 0.80 1.23 428.75
250 1ml 20.07 56.50 181.51
Table 5 gives a comparative sales rise of
5 mg
10T 1.05
— 7.15 581.90 Testanon -25
1ml 1.45 10.40 602.70
drugs acting on musculo-skelctal disorders. Rise less than 50 per cent (3) ,
50 1ml 6.54 .18.70 ■ 181.63
Rise less' than 50 per cent (1) . . o •. r •.■iZfcZ
In this group, the non-steroidal anti Rise 50-100 per cent (I) Oral Contraceptive • ~f T. : • •
inflammatory drugs (NSAIDs) have shown Antidepressants I0T 6.00 23.90 298.33 Lyndiql
’ 22T 4.88 14.40 .. 207.69
Doxetar 25 mg
a minimum rise of 46 per cent because most Surmontil
10 mg JOT 2.14 5.52 157.94 Corticosteroids
of these NSAIDs are not widely used and
1 ml 7.37 32.50 340.98
11.10 161.18 Dacabolin
25 mg 10 T 4.25 ..................
10 T 2.20 5.90 . 168.18 Kenacort lOmg 1ml 8.90.18.19 1 04.38
these are replaced by. newer and costlier Tancodep
10T 1.86 4.15" 123.12
Walacort
drugs; Muscle. relaxants have shown an Antiemelic
4T 0.74 2.74 270.27 Wysolon 5mg 10T 2,15 . 4.94 129.77
appreciable rise of 281 percent which means Avomine
4T 0.88 3.10 252.27. Gonadotrophin
Diligan
amp 77.10 163.00 111.41
that the prices have multiplied nearly four Dramamine
10 T 4.86 10.45 115.02 FSH
times. Carisoma shows a rise of 512 per cent Maxeron
60
1 ml 5.00 13.04 160.80 Gonadotrophin
16.01 51.00 21,8.55
LH
1000x3
7.95
305.61
1.96
lOt
siquil
10
mg
and it has further increased the price during
less than 50 per cent (7) I ml 0.84 4.93 413.54 Rise
3 mg
1995-96. Carisoma contains carisoprodol
Rise 50-100 per cent (5)
T
ot 0.88 7.08 '704.55
Stemetil
5
mg
?
/
and British National Formulary (No ’29,
> 25 mg
10 T 1.40 11.31 707.85 Reduced (3) , . .( *
March 95, page 402) says "The clinical inj
' . ■ I ml 0.70 4.30 514.29 Hypoglycemics
Chlorformin . 10T 1.40 2.90 107.17
efficacy of carisoprodol as muscle relaxant Rise less than 50 per cent (6)
Copamide’
10T 1.33 4.00 '■ 200.75
'
is not well established although it has been Rise 50-100 per cent (5)
DBI
25T 5.44 14.95 174.82
Daonil
.
10T ■ 1.71 3.54 107.02
included in compound analgesic preparations Anticonvulsants
Dilantin
HOT 11.73 44.41 . 278.60
Diabinese 100 10T 1.06 4.79 351.89
(Carisoma compound)”; Epidosin is an anti Epsolin
100 T 6.42 44.29 589.88
250 10T L59 7.60 377.99
spasmodic and is useful in visceral spasms Eptoin •
100 1 7.37 37.00 402.04 Euglucon ,
JOT .1.72 ■ 3.54 105.81
101 1.36 5.59 311.02 Insulin Soluble 10ml 11.10 33.50 201.80
and it shows a rise of427 percent. Carisoma •Garoin
10ml 12.00 31.50 162.50
compound, an analgesic.combination, has Mazetol 200 mg 10 T 8.06 17.96 122.83 . Lente
. 100T 31.12203.00 552.31
NPH ' .
10ml 11.64 31.50 170.62
•increased by 299 per cent, Parafon (a brand Mysoline
Rise less than 50 per cent (1)
Restihon0.5
10T 1.13 6.50 475.22
of chlorzoxazone) ia rise of 283 per cent, Rise 50-100 Percent (2) . .
Rise 50-100 per cent (3)
Robinax (a brand :methocarbamox,4he Rigidity and tremor controller Ketnadririe
Thyroid and antithyroid drugs
2.5mg
100T 11.78 71.10 503.57 Eltroxih
100T 2.74 20.40 644.53
.efficacy of which is not established) tablet
5.0mg '’
100T 22.38 131.50 487.58 Neo Mercazole 100T ■ .15.97 -73.24 .358.61
and injectible by 245 per cent. Rubefacients
50T. 44.03 134.50 205.47 Proloid
50T 8.14 76.00 .833.66
are topical analgesics that principally act by Levopa
221.33
Pacitahe; •
10T 1.04 -7.40 611.54 Total .
causing irritation. These as a total have
:
2334
'■ ■ ■■
Economic and Political Weekly
-.'■■. ...J
August 24-31, 1996
i
r
Table7:’GenhoUrinary
Product
Antibiotics
Achromycin
Diuretics
Hythalton
Lassix
Reduced (3)
Rise less than 50 per cent (2)
Rise 50-100 per cent (3)
Urinary anti-infectives
Gramoneg
Mandelamine
0.5g
1.0g
Pyridium
Wintomylon
Rise less than 50 per cent (1)
Rise 50-100 per cent (3)
Product
Antibiotics .
Achromycin
Althrocin 250mg
Chewable
Bacipen
Bistrepen
Broacil
Compicillin 250
500
Cephaxin 250mg
500mg
Chloromycetin
Dicrystacin s
Eltocin
Erythrocin
lOOmg
250mg
Klox ,
Paraxin 250mg
500mg
Penidure LA 6
LA 12 ,
LA 24 •
PenivoraJ
Pentids 200
400
800
Rector 250mg
500mg
Restecliri 500
Subamycin250
500
Synthocillin 250
Threocyclin;7.
50mg
Thromycin
Prices reduced (8) .
Rise less than 50 per cent (7)
Rise 50-100 per cent (23)
Sulphonamidesf..
Madnbon. . . ; ,
Pack
1980
4C
2.32
10T
2 ml
3.74
0.86
46.68
14.93
10T
14.49
30T
15T
10T
8T
8.12
7.82
2.74
,14.92
Pack
1980
Prices
1995
PerCent
Rice
product'
Local and systemic drugs
4.82 107.76 , Betadine , .
Dicnoestrol
Hamycin
Triple sulpha
26.04 596.26
Rise less than
2.02 133.72
50 per cent (4)
30.19
Rise 50-100 per cent (1)
22.22 <
Drugs acting on Uterus
Ingagen M
46.80 222.98
Pitocin
26.04 220.69
Rise less than
50 percent (1)
26.06 233.25
14.50 429.20 ■ Rise 50-100 per cent (1)
Spermicidal
44.00 194.91
Del fen c applicator
Total
. u
■ Pack
1980
■_
Prices
1995
Per Cent
Rice
100ml
50g
10ml
"30g
15.00
5.25
2.34
4.99
24.60
64.80
14.65
24.75
130.67
1134.29
526.07
395.99
1ml
I0T
lamp ‘
1.34
5.16
1.05
6.13
21.29
■' 3.40
357.46
312.60
223.81
6.29
33.47
432.11
131.64
Table 8: Infection
Pack'
Prices
Per Cent
Per Cent Product
1980
Rice
1995
1995
Rice
Rise less than 50 per cent (9)
.A
' '
Rise 50-100 per cent (1)
4.82 107.76
Reduced (9)
36.01
141.52
■■■■•r •» .
■ ,■;
Antiluberculours
15.52 139.51
vial
1.03
Ambistryn-S 0.75
5.74 457.28
256.00 167.50
1.18
1.00
vial
7.10 501.69
8.98 701.79
10T
1.78
3.90 110.10
24.44 398.78
Erbazide 400
100T
13.77
38.50 179.59
INA-PAS
9.85 113.20
granules
100g
13.51
38.50 184.97
26.25 110.00
11.87 163.78
100T
4.50
49.60 121.33 . Isonex 100
3.41
30T
9.75 185.92
. 300
20.27 137.35
5.96 142.28
Strepto-erbazidc
vial,
22.46
19.60 110.25
244.28 147.48
Reduced (3)
37.00 11143
Rise less than 50 per cent (4)
24.00 467.38
Rise 50-100 per cent (4)
27.25 467.94
Antileprotics
4.67 285.95
Hansipran 100
100T 1184.50 180.00 reduced
•21.98 107.36
Antifungals
2.34
14.65 526.07
10ml
Hamycin
13.48 189.89
14.29
63.13 ' 341.78
I2T.
33.10 209.64 . Mycostatin
14.36 108.12 ; Rise 50-100 per cent (1) .
.. .
Antiamoebics
11.67 233.43
Dihydroemetin
12.96 207.84
,30mg 1ml 1.27
4.13 225.20
7.00 222.58
6.01
194.61
60mg 2ml 2.04
11.82 211.05
6.57 257.07
1.84
20T
Enteroquinol
19.94 197.61
10T
14.90 108.99
7.15
Monizole 600mg
2.95 109.22
4.06
3.75
Reduced (1) ..
,
5.27 184.18
Rise less than 50 per cent (3)
11.00 213.39
Rise 50-100 per cent (2)
13.40 264.13
Antimalarials'
r
5.51; 115.2'3
3.24 208.57
4T
1.05
Nivaquin
13.04; 240.77
1.93 . 164.38
2ml
0.73
7.79 109.41 ■
L
10T
1.99
8.10 • 307.04
Rcsochin
14.03 119.91
Rise 50-100 per cent (1)
7.42 ’ 113.22
Aritihelmintic ■
10.73 114.60
1.00
. 2.27 127.00
..IT
Vermisol
Rise less than 50 per cent (5)
8.23 129.25
Rise 50-100 per cent (4)
8.47 Ulis
’. ”
Vaccines
26.43 123.98
10ml
18.35 126.00 . 586.65
, Anti-snake venom
76.22
5ml
16.00
34.88 118.00
• Diphtheria Antitoxin
Gas gangrene ’
32.23 193.00
antitoxin
11.00
66.49
Total '
11.95 195,06
Prices
4C
10T
I0T
IOOT
vial
5 dose
'4C
IOC
IOC
60ml
. 4C .
4C
12C
60ml
1 dose
60ml
2.32
14.91
6 48
95.70
1.12
4.90
4.62
12.50
22.41
8.54
9.30
17.50
4.23
4.71
1.21
10.80
10T
10T
24g
IOC
6C
vial
vial
vial
6T
6T
6T
4T
6C
6C
4C
12C
4C
4C
4.65
10.69
6.90
3.50
4.21
2.17
3.80
6.70
1.41.
1.96
3.51
3.68
2.56
3.83
3.72
6.38
3.48
5.00
10ml
6C
IOC
3.59
3.99
i 1.80
108.50
10T
4.05
' •
Economic and Political’ Weekly • August ,24:31,-1996
■
'
:
.
. V.
2335
Table 10: Respiratory Products
Product
Pack
Price Per Cent
1980 1995 Rise
Respiratory stimulants
Rise less than
100 per cent (1)
17.50 32.87
Bronchospasm ;relaxant
Allupent
I ml 0.89 4 12 362.92
100ml 6.77 38.06 462.19
20T 5.76 24.60 327.08
Asmapax depot 12T 2 60 8.12 212.31
Asmotone
110ml 5.57 18.17 226.21
Autohaler 200dose 27.95 74.32 165.90
Marax
20T 5.13 26.45 415.59
Sedonal
10T 1.11 3.41 207.21
Tcdral
10T 1 76 3.90 121.59
Tcdral SA
10T 2 23 9.75 337.22
Reduced(2)
6.71 4.54
Rise less than 50 per cent (2)
Rise 50-100 per cent (1)
Expectorants
Actifed
I0T 1.90 8.15 328.95
Benadryl exp 114ml 5.23 21.00 301.53
Cinaryl
60ml 3.83 10.60 176.76
I0T 2.12 5.40 154.72
Clistin
115ml 7.65 28.45 271.90
Corex
50ml 4.08 8.63 112.56
Cosavit
10T 1.30 3.19 145.38
Coscapin
115ml 6.18 18.00 191.26
Cosome
140ml 6.71 35.43 428.02
Dilosyn
120ml 5.71 16.22 184.06
Dristan
60ml 4.38 9.85 124.89
10T 1.70 3.65 114.71
Exiplon
50ml 4.00 11.58 189.50
Grilinctus
100ml 6.07 17.60 189.95
Karval Inhaler 12C 6.25 16.20 159.20
Phensedyl
125ml 5.91 14.92 152.45
100T 33.20 250.00 653.01
Selvigon
10ml 5.76 34.14 492.71
Selvigon exp 100ml 6.36 31.72 398.74
Sudafed
115ml 4.30 12.71 195.58
10T 0.92 4.87 429.35
Tixylix
125ml 5.16 12.13 135.08
Triominic
100ml 5.85 26.67 355.90
Tristina
110ml 3.94 19.52 395.43
Tuxyne
10T 1.59 7.96 400.63
Zeet exp
110ml 4.41 19.58 343.99
Rise less than 50 per cent (I)
Rise 50-100 per cent (4)
Total
258.45
Table 11: Ear, Nose and Oropharynx
Products
Product
Price PerCent
1980 1995 Rise
Local reactants on nose
Efcorlin Nasal 15ml 3.51 8.40 139.32
Endrine
30ml 5.47 20.22 269.65
Emdrine mild 30ml 5 47 18.93 246.07
Fenox
15ml 2.46 14.50 489.43
Kemicetin
Antiozona
10ml 6 02 22.00 265.45
Nasivin
10ml 4.50 16.00 255 56
Otrivin
10ml 4.75 15.65 229.47
Otrivin
paediatric
10ml 4.19 13.80 229.36
Rise less than 50 per cent (1)
Rise 50-100 per cent (1)
Oropharyngeal preparations
Rise less than
100 per cent (2)
9.75 17.08
Aural preparations
Chloromycetin 5ml 2.05 6.67 225.37
Dexona eye/ear 2.5ml 3.30 7.46 126.06
Neosponn-H
5ml 6.89 14.81 114.95
Paraxin ear
6ml 2.83 8.04 184.10
Waxolve
10ml 4.31 13.50 213.23
Rise less than
50 percent (5)
27.71 36.81
Total
142.22
Pack
Economic and Political Weekly
whereas the remaining 12 products have
increased the prices ranging from 182 per
cent to 1681 per cent. Most of these drugs
are needed by women. Among oral
contraceptives only Lyndiol has shown a
rise of 208 per cent. Among corticosteroids
three products have shown a decrease in
prices with the maximum rise claimed by
Docabolin. Hypoglycemics, used for
Table 12: Eye Products
Product
Price Per Cent
1980 1995 Rise
Anti-infective preparations
Albucid
20 per cent
14ml 3.73 7.60 103.75
30 percent
14ml 3.83 7.83 104.44
Chloromycetin
applicap
50 5.78 17.60 204.50
clearin
10ml 4.25 20.00 370.59
Kemicetin
3g 1.55 4.95 219.35
Locula
10 per cent
10ml 2.22 6.65 199.55
20 percent
10ml 2.61 7.60 191.19
30 per cent
10ml 2.84 8.55 201.06
Paraxin
softicaps
50 4.93 17.60 257.00
5g 1.76 4.23 140.34
Proto Boric
10ml 3.50 7.00 100.00
Vanmycetin eye 5ml 2.29 5.21 127.51
Reduced (2)
Rise less than 50 per cent (2)
Rise 50-100 per cent (1)
Anti-inflammatory and antiallergicpreparations
Chlorocort
applicaps
50 7.74 17.40 124.89
Dexona eye/ear 2,5ml 3.30 7.46 126.06
Glaucoma
Biomiotic
10ml 6.75 18.80 178.52
Carpo-miotic
0.5 per cent 10ml 5.60 12.72 127.14
4 per cent
10ml 8.85 23.85 169.49
Pilocar
2 per cent
5ml 3.81 15.90 317.32
4 per cent
5ml 4.57 26.29 475.27
Mydriutics and cycloplegica
Less than 100 per cent (3)
Diagnostic and miscellaneous preparations
29.79 109.30 266.90
Catalin
less than
100 per cent rise (2) 8.75 12.00
Total
150.05
Pack
Table 13: Drugs for Allergic Disorders
Product
Pack
Price PerCent
1980 1995 Rise
Antiallergic drugs
25T 2.34 15.50 562.39
Benadryl 25g
114ml 4.81 13.25 175.47
20T 5.20 17.72 240.77
Calciluvin
100ml 7.07 17.48 147.24
10T 1.38 5.82 321.24
Dilosyn
120ml 5.04 17.74 251.98
10T 1.00 4.80 380.00
Foristal
10T 1.57 7.50 377.71
-lontabs
Hepasulfol AA 25T 4.96 30.72 519.35
10T 1.70 5.50 223.53
Incidal
12T 3.15 11.40 261.90
Mebryl
Phenergan 25mg 10T 0.84 1.93 129.76
125ml 3.80 9.37 146.58
Vallergan fort 50ml 3.42 23.04 563.98
Rise less than 100 per cent
263 89
Total
August 24-31, 1996
treatment of diabetes have a total rise of 147
per cent. Maximum rise is registered by
Diabenese 100 mg-352 per cent and 25 mg
-378 percent, and 475 percent by Rastinon.
Insulins have registered a rise of 170 per
cent, but now the use is shifted to a costlier
brand of purified human insulin.
In the genito-urinary group of drugs
diuretics have shown a decrease in total
price by 3 per cent, whereas the drugs acting
on the uterus by 23 per cent. Spcrmicidals
have registered a rise of 432 per cent, local
and systemic drugs by 221 per cent and
urinary antiseptics by 171 per cent.
Table 7 gives the comparative price rise
of drugs acting on thegenito-urinary system.
The total rise is only 97 per cent because
diuretics have shown a decline of 3 per cent
and drugs acting on the uterus, a decline of
23 per cent. Deflen a chemical barrier for
contraception shows a rise of 432 per cent,
closely followed by local and systemic drugs
by 221 percent and urinary antiseptics by
171 per cent. In the diurectics Hythalton
shows a rise of 596 per cent and Lassix a
rise of 134 per cent. In the urinary antiinfectives, Pyridium, aurinary tract analgesic
shows a rise of429 percent. This has further
shown a rise of 51 per cent, making a total
riseof 699 per cent since 1980. Mandelamine
shows a rise of 233 per cent, Gramoneg 223
per cent and Wintomylon 195 per cent.
Among local and systemic drugs Dienestrol
cream has taken a rise of 1,134 per cent,
Hamycin by 526 per cent, Triple Sulpha
cream by 396 per cent. Ingagen-M (a rise
of 357 per cent) and Pitocin (a rise of 224
per cent) are drugs used during labour. Thus
all the products in this group take a rise of
132 per cent.
:.
Table 8 shows a total rise of 59 per cent
in the case of anti-infective drugs because
there is a reduction in the prices of
sulphonamides, anti-TB drugs, anti-leprotic
drugs. In the sulphonamide group the popular
brands of Septran and Bactrim tablets prices
have reduced, but the suspension and syrup
prices have marginally increased.
Antibiotics show a rise of 106 per cent,
wherein the maximum rise is shown for
Bistrepen (702 per cent). This is a
combination of Streptomycin and penicillin
and a demand is being made to ban this
combination. Chloramphenicol brands have
shown a rise as Chloromycetin 467 per cent,
Paraxin 233 per cent and Reclor 241 per
cent. Erythroxycin a brand of erythromycin
100 mg shows a rise of 190 per cent, while
250 mg shows a rise of 210 per cent.
Gentamycin (Biogracin, Garamycin,
Genticyn), Doxycycline (Duracycline,
Lydox) show a decline in prices. Klox a
brandofcloxacillin (injectable) has registered
a price decrease and for capsules a price rise
of 96 per cent and syrup by 108 per cent.
Long acting penicillinPenidurLA 6 increased
2337
by 223 per cent, LAI2 by 211 per cent and
LA24 by 198 per cent. Penlids, a brand of
oral penicillin has gone up by 264 per cent
for 8 lac units. 213 per cent for 4 lac units
and 184 per cent for 2 lac units. All others
are in the range 100 to 150 per cent rise.
Among anti-tubercular drugs there is a
total reduction of 0.66 per cent and that of
anti-leprotics of 2.44 percent. The reduction
in anti-tubercular drugs is due to drastic
reduction in price of Tibrium (rifampicin).
Ambistryn, the brand of streptomycin
injection has gone up by 502 per cent for
1 g vial and 457 per cent in 0.75 g vial. Other
drugs (Erbazide 119 percent, Ina-Pas tablets
180 percent, granules 185 percent, Isonex
100 mg 164 per cent, and 300 mg 186 per
cent) have shown price increses, but these
drugs are hardly used now. In the antiTable 14: Skin Products
Product
Pack
Price I?er Cent
1980 1995 Rise
Soothing and protective
Caladryl
57 m! 2.64 15.10 471.97
K-Y Jelly
85g 5.35 52.78 886 54
Siloderm
20g 3.33 18.02 441.14
Keratolytic and cleansers
Cotaryl
50g ' 6.03 24.90 312.94
Derobin
25g 3.46 12.85 271.39
K 5 Hair tonic 100ml 15.00 67.29 348.60
Keralin
5g 2.00 4.23 111.50
Topical non-steroid and anti-pruritic
Crotorax
20g 4.08 13.91 240.93
Topical antifungal and antiinfective
Ascabiol
50ml 2.53 9.30 267.59
Dermoquinol
4 per cent
25g 2.32 6.86 195.69
55m! <99 13.15 171.54
Emscab
Multifungin
30g 4.92 14.78 200.41
30ml 5.02 14.78 185.88
Mycostatin
10g 3.99 16.50 313.53
Pragmatar
25g 4.35 12.00 175.86
Rise less than 50 per cent (1)
Rise 50-100 per cent (2)
Antiinfective
Bctadine
10g 4.90 11.00 124.49
100ml 15.00 34.60 131.00
Dettol
55ml 2.40 7.75 222.92
-cream
25g 2.44 4.97 103.69
-obstetric
500g 15.77 40.84 158.97
Furacin
28g 4.60 11.30 145.65
Iteol 3
115ml 3.21 7.95 147.66
Nebasulf skin
15g 4.23 13.02 207.80
powder
10g 3.37 10.95 224.93
Ncosporin
10g 4.98 10.81 117.07
5g 3.19 ’ 6.50 103.76
Soframycin
15g 3.74 9.03 141.44
Topical Steroid Preparation
Ultralan
5g 7.62 42.46 457.22
Rise less than 50 per cent (2)
Rise 50-100 per cent (3)
Miscellaneous
Hirudoid
14g 7.70 32.00 315.58
Manaderm
40T 8.39 22.50 168.18
Mascoraleri
20T 7.15 23.02 221.96
Messe cream
25g 4.52 47.20 944.25
Melanocyl
40T 16.57 35.84 116.29
Paraminol
25g 4.15 11.20 169.88
Thrombophob 15g 4.18 14.40 244.50
Rise less than 50 per cent (2)
Rise 50-100 per cent (3)
Total
297.06
2338
leprotics the priccof Hanscpran (Clofazimine
100 mg) has marginally reduced (from
Rs 184.50 to Rs 180.90), but has gone up
to Rs 211.20 during 1995-96. Anti-fungals
have shown a big rise of 259 per cent and
Hamycin shows 526 per cent, Mycostatin
342 per cent. In the anti-amoebics the price
of Aristogyl (metronidazole 200 mg) has
been reduced from Rs 4.06 to Rs 3.75 and
has increased to Rs4.63 during 1995-96. The
price of Dehydroemetin 30 mg injection
increased by 225 per cent, 60 mg by 195 per
cent; Enteroquinol hasshown aprice increase
by 257 per cent and thus a total rise of 71
per cent. Among anti-malarials Nivaquin
tablets has shown a price increase by 209
per cent and injection by 164 per cent. Prices
of Resochin have gone up by 307 per cent
and the total rise is 192 per cent. Among
vaccines there is big rise in anti-snake venom
by 587 percent, Diphtheria antitoxin by 118
per cent and gas-gangrene antitoxin by 193
per cent, taking the total rise to 326 per cent.
Table 9 on nutrition shows a total rise of
225 per cent. Maximum rise of 659 per cent
is seen in food products, minerals and
nutritional additives with 229 per cent,
anabolics by 221 per cent, drugs for obesity
by 193 per cent, vitamins by 184 per cent,
iron preparations by 155 per cent and tonics
by 148 per cent. Bayer’s tonic shows a rise
of 317 per cent and further increased in
1995-96 to a total rise of 508 per cent.
Kinctone by 306 per cent and Ncogadine by
273 per ent. In the iron preparations
Dcxorange by 360 per cent and further rise
during 1995-96 making a total rise of 427
per cent. Hepatoglobin by 304 per cent and
Iberol liquid by 302 per cent. In the minerals
Table 15: Products for Metabolic disorders
Pack
Price Per Cent
1980 1995 Rise
Corcinochemdtherapeutic
Alkeran 2mg 25T 37.88 192.70 408.71
5mg 25T 62.45 337.89 441.06
Blcocin 15mg
162.70 953.68 486.16
Endoxan
50T 44.68 122.70 174.62
lOOmg
vial 5.17 11.77 127.66
200mg
vial 8 31 18.70 125.03
500mg
'vial 18.32 39.06 113.21
Leukeran 2mg 100T 25.58 518.12 1925.49
5mg 100T 58.64 566.76 866.51
Leunase
vial 97.91 225.00 129.80
Mitomycin-C 2mg
17.40 54.38 212.53
Mustine
vial 16.20 112.60 595.06
Mylcran 2mg I0OT 13.85 260.12 1778.12
Puri-Nethol
25T 22.79 201.18 782.76
Reduced (I)
69.50 48.00
Rise less than 50 per cent (2)
Itntnunosuppresanls
Imuran 50 mg IOOT 271.67 878.60 223.41
Gout
Reduced (1)
Poisoning and metabolic dysfunction
BAL
2ml 7.75 38.50 396.77
Glutaneurol 100T 12.01 78.40 552.79
Rise 50-100 per cent (I)
Total
335.6!
Product
and nutritional products Calcium Sandoz
injectable with a riscof 614 percent, followed
by Hermin liquid by 433 per cent, Eleclral
by 320 per cent, Calcinol-F by 409 per cent,
Sharkoferol by 299 per cent. The total rise
is 228 per cent. Vitamin products show a
rise of 184 percent, with vitamin A products
like Aquasol tablet going up by 390 percent,
injectables by 334 percent, Arovit tablet by
394 per cent, injection by 212 per cent, and
drops by 489 percent. Vitamin C prices have
gone up - Cecon 500 drops by 285 per cent.
Celin 500 mg tablet by 295 per cent, 100
mg 346 per cent, Citravite tablets by 413
per cent, Redoxon 500 mg tablet by 252 per
cent, Sukcec tablets by 465 per cent and all
other combination vitamin products by 100
to 300 per cent. There were only two anti
obesity products, viz, Flabolin that has gone
up by 108 per cent whose prices have further
risenby31 per cent in 1995-96 and Ponderax
by 297 per cent. Anabolics, though really
not needed have shown a rise of220 percent.
Durabolin 25 mg has gone up by 272 per
cent, Deca-Durabolin by 171 per cent and
Orabolin by 266 per cent. Strangely enough
food products have shown an overall totaj.
rise of 659 percent, wherein Protinex shofl
a rise in price of 488 per cent - Protinules
277 per cent, Sanatogen 887 per cent and
Syu 316 per cent. The prices of all these
products have further risen during 1995-96.
Table 10 gives the details of respiratory
products, that show an overall rise of 202
percent. Marax, a combination of ephedrine,
theophyline and a tranquiliscr shows a rise
of 416 percent, Allupent injeclible 363 per
cent, the syrup by 462 per cent and tablets
by 327 per cent. Tedral SA by 337 per cent
and others ranging from 122 to 212 per
cent. Expectorants show a rise of 300 per
cent (that is four times) Selvigon tablets
653 per cent, drops 493 per cent, Cosome
428 per cent Tuxyne tablets 401 per cent,
Sudafed tablets 429 per cent, and Actifed
329 percent. Among liquid cough mixtures
Benadryl expectorant shows ariseof 301.53
and has further gone up during 1995-96
- Selvigon by 399 per cent, Triominic by
356 per cent, Tristina by 395 per cent
Zeet by 344 per cent.
Table 11 gives the details of products used
in ENT specialities-with a total rise of 217
per cent, and the maximum rise being in
Fenox nasal drops by 489 per cent, throat
products by 75 per cent. Ear drops show a
total rise of 85 per cent, Chloromycetin by
225 per cent and Waxolve by 213 percent.
Tabic 12 shows the price rise of eye
ointments and drops. Anti-infectives have
shown a total rise of 80 per cent chloramphenicol preparations have shown
a big rise of 204 per cent for Chloromycetin
applicaps (further increased by 21 per cent
in 1995-96), Paraxin applicaps 257 percent.
Another anti-infectivesulphacetamidedrops
Economic and Political Weekly
August 24-31, 1996
has gone up - Albucid 104 per cent (1980-96
- 186 per cent), Locula (30 per cent by 201
percent (1980 to 96-298 per cent). Clcarine
a brand of naphazoline anti-inflammatory
shows rise of 371 per cent. The drops for
glaucoma have shown a big rise of 161 per
cent - Carpo-miotic a brand of pilocarpine
shows a rise of 354 percent, Pilocar by 475
per cent. Biomiotic, a combination drug has
gone up by 179 oer cent-in 1995-96 it has
further gone up by 113 per cent making for
an overall rise of 493 per cent
Table 13 gives us a comparative picture
of anti-allergic drugs. There has been a total
rise of 264 per cent in their prices. Vallergan
forte - a rise of 564 per cent, Bendaryl
capsules of 562 percent, Hepasulfol of 519
per cent, Foristal of 380 per cent, Dilosyn
of 321 per cent, Mebryl of 262 per cent,
Calculuvin by 241 per cent, and others. The
prices of Phenergan tablets had gone up by
130 per cent and syrup by 147 per cent.
During 1995-96 Pheneragan tablet price have
risen from Rs 1.93 to Rs 4.35 and syrup from
Rs 9.37 to Rs 14.52 thus making a total rise
of 617 per cent for tablets and 282 per cent
for syrup. Prices of Benadryl, Calciluvin and
. Dilosyn have further risen during 1995-96.
Table 14 gives the price rise in skin
ointments by 181 per cent. K Y sterilised
lubricating jelly used for rectal and vaginal
(internal) examination shows a rise of 887
per cent and has further risen in 1995-96
making a total rise of 1203 percent, Caladryl
by 472 per cent and Siloderm by 441 per
cent. Both these product have further risen
during 1995-96. In the keratolytics price of
K-5 hair tincture has give up by 349 per cent,
Cotaryl by 313 per cent and Dorabin by 271
percent. In the anti:fungals Mycostatin shows
a price rise of 314 per cent, Ascabiol of 268
per cent, Multifungin of 200 per cent and
Pragmatar of 176 per cent. The remaining
three products show a rise of less than 100
per cepl..
Among anti-infectives (antiseptics), there
is a total rise of 149 per cent - the maximum
rise of 225 per cent is in Nebasulf ointment,
223 per cent for Dettol liquid, and all others
100 to 158 per cent. Prices of all these
products have increased during 1995-96.
The steroids products have shown a price
rise from 50 to 100 per cent and two less
than 50 per cent - Ultralan, a brand of
fluocortolon shows a rise of 457 per cent.
In the miscellaneous skin preparations
Hirudoid shows a price rise of 316 per cent.
The maximum price rise appears to be in
drugs used in metabolic disorders, i c, in
cancer - the total rise is 336 per cent.
Particularlycarcino chemotherapeutic agents
(cancer drugs) show a total price rise of 417
per cent. Puri-Nethal, a drug for acute
leukemia (blood cancer) shows a price rise
of783 percent, Leukeran, adrug forovarian
and breast cancer of 1925 per cent (it has
Economic and Political Weekly
further risen during 1995-96 by 106 per
cent, making a total rise of 4066 per cent),
Myleran, a drug for leukemia shows a
rise of 1778 per cent (and a further rise
in 1995-96 making a total rise of 2292 per
cent), Mustinc, with a rise of 595 per cent.
The only price reduction is in Vincrislin.
Among immunosuppresants, Imuran, a drug
used for rheumatoid arthritis unresponsive
to other drugs, shows a price rise of 223 per
centwithmarginal 13.35 percentriseduring
1996. The price of Zyloric, the only brand
of allopurinol for'gouty arthritis has been
reduced from Rs 57.88 to Rs 12.04, a
rcduclionof 79 percent. Again theemergcncy
drugs used for treatment of poisoning show
a total price rise of 351 per cent. Strangely
enough Glutanctirol (a brand of L-glutamic
acid, reference of which is not found in BNF
and only a passing reference in Martindale)
shows a price rise of 553 per cent. BAL
injection is used for metal poisoning and it
shows a price rise of 397 per cent. Most of
the drugs for metabolic disorders are i mponed
and particularly those fortreatmentof various
types of cancers and for treatment of poison
ing should be reasonably priced. Though
these drugs may not be falling within the
DPCO criteria, the government mustsee that
these emergency drugs are made available
at reasonable rates and see that the prices
do not go on increasing five to 40 times.
DEVELOPMENT, DISPLACEMENT AND
REHABILITATION
June 15, 1996
Draft National Policy for Rehabilitation: Objectives and Principles
B K Sinha
Economic Perspectives on Resettlement and Rehabilitation
Sangeeta Goyal
Involuntary Resettlement: Survey of International Experience
Poli Asthana
Whose Nation?: The Displaced as Victims of Development
Smitu Kothari
Displacement and the Law
Usha Pamanathan
Vasava Identity in Transition: Some Theoretical Issues
Poxahne
Hakim
Development, Displacement and Rehabilitation: Locating Gender
Enakshi Ganguly Thukral
Mental Health Consequences of Displacement and Resettlement
Byron J Good
Dislocation and Rehabilitation: Defining a Field
Veena Das
Public Policy Responses to Development-Induced Population
Displacements
Michael M Cernea
Displacement due to Mining in Jharkhand
Mathew Areeparampil
Development Projects, Displacement and Outcomes for Displaced:
Two Case Studies
S Parasuraman
Machkund, Upper Kolab and NALCO Projects in Koraput District, Orissa
William Stanley
Tribal Resistance in the Chhechhari Valley: A Field Report
Nita Mishra
Draft National Policy for Rehabilitation of Persons:
Displaced as a Consequence of Acquisition of Land
August 24-31, 1996
Ministry of Pural Development
For Copies, Write to:
Circulation Manager
Economic and Political Weekly
Hitkari House, 284 Shahid Bhagat Singh Road,
Mumbai 400 001
2339
VHAI-AIDAN-NCCDP-RDCC DRUG CONSULTATION
ON
IMPACT OF, POLICY CHANGES ON DRUG POLICY AND DRUG USE
26,
27 August
1996
INDIA'S FORGOTTEN PEOPLE AND THE BREAKDOWN OF
THE
PUBLIC HEALTH SYSTEM
A PRESCRIPTION FOR THE MALADY
DEEWBAR BPNERJI
Professor Emeritus
Jawaharlal Nehru University
a. nd
Nucleus far Health Policies and Programmes
B~43f Panchsheel Enclave
New Delhi 110017.
CONTENTS
PREFACE
PART I
THE RESEARCH PROBLEM AND THE METHODOLOGY
1.
2.
The Research Problem.
The Methodology.
PART II
THE FORGOTTEN PEOPLES OF INDIA
Interaction of the two classes.
Struggle Between Memory and Forgetfulness.
The forgotten Peoples and their Size and
Distribution Over Time.
6. Remembering the Forgotten Peoples:
Portraits of Some Families.
7. Population Growth as a Function of Human Ecology.
8. Environmental Conditions of Living and Working.
9. The Health Problems.
3.
4.
5.
PART III
INTERFACE BETWEEN PEOPLE AND PUBLIC HEALTH INSTITUTIONS
10. People's Response to their Health Problems.
11. A Conceptual Basis of Public Health and Public Health Servic
es.
12. An Overview of the Sickness of the Public Health
Servic
es.
13. Nationwide Surveys of Utilisation of Curative
Health
Services in Rural Areas.
14. The National Family Health Survey.
15. Utilisation of Some Health Services in Urban Populations.
PART IV
PERFORMANCE OF THE "FRONT LINE" PUBLIC HEALTH
INSTITUTIONS
16. Impact of the Family Planning Programme on the Public Health
Services.
17. Performance of the Health Service Aspects of Family Planning
in Primary Health Centres.
18. Health Services for Women.
19. The State of the Primary Health Services in India.
20. A Twenty-three Year Account of the Changes in Eleven Primary
Health Centres and their Perception by Local Village Communities.
21. Access of Public Health Services to Some Specially Disadvan
taged Groups.
22. The Kerala and the Tamil Nadu "Models".
23. Hospitals and Other Medical Care Institutions.
24. Issues of Efficiency and Equity in Public Health Services.
25. Summing Up: The Forgotten Peoples and the Breakdown of the
Public Health Services.
1
' PART V
NATIONAL HEALTH PROGRAMMES
-26. The Making of the Theory and Practice of a "New Public
Health": The Case of India's National
Tuberculosis Programme.
27. Malaria as a Public Health Problem.
28. The Universal Immunisation Programme.
29. The National AIDS Control Programme.
30. Leprosy as a Public Health Problem.
31. Blindness as a Public Health Problem.
32. An "Epidemic of Epidemics" Breaks out as the Public Health
Service System Crumbles.
33.Nutrition Programmes.
PART VI
ADMINISTRATION OF THE PUBLIC HEALTH SERVICES
34. Quality of the Health Administration.
35. Domination of the Health Services by Generalist Administra
tors.
36. Regional Variations in the Public Health Services.
PART VII
HEALTH MANPOWER DEVELOPMENT
37. Sociology of the Trends in Medical Education.
38. Decay of the Medical Education System.
39. Abdication of Responsibility by the Guardians of Medical
Education.
40. Panorama of Changes in Medical Education.
41. Machiavelli Replacing Hippocrates: Issues of Medical Ethics in.
Medical and Public Health Practice.
42. The Nursing Profession and Nursing Practice.
43. Human Resources From Other Health Professions.
44. Human Resources in Terms of Paramedical Personnel.
45. Health Systems Research for Development of Human Resources for
Health.
PART VIII
STATE OF THE KEY PUBLIC HEALTH INSTITUTIONS
46. Decline of the Key Public Health Institutions.
47. The
Indian Council of Medical Research.
48. The All India Institute of Medical Sciences.
49. The National Institute of Health and Family Welfare.
50. The .All India Institute of Hygiene-and Public Health and the
National Institute of Communicable Diseases.
PART IX
QUESTIONABLE INVOLVEMENT OF ECONOMISTS IN PUBLIC HEALTH
PROGRAMME AND POLICY ANALYSIS
51. Ethical Issues in the Practice of Public Health Quackery by
Economists.
52. Impact of the Structural Adjustment Programme on State and
Central Budgets.
53. Promotion of Curative Allopathic Medical Services Through the
Private Sector.
PART X
OTHER ASSOCIATED AREAS IN HEALTH ADMINISTRATION
54. Policy on Drugs.
55. Promotion of the Indigenous Systems of Medicine and their
Place in the Public Health Service System.
56. Voluntary Health Work and Foreign/International
Organisation/Governmenr Driven Non-Governmental Organisations.
PART XI
THE INTERVENTION OF FOREIGN AGENCIES IN PUBLIC HEALTH
PROGRAMMES IN INDIA
57. Volte-Face by the »orld Health Organisation and the United
Nations Children's Fund.
58. Imposition of Technocentric Programmes by the North on the
South.
59. World Bank's India Population Projects I and II and Other
Foreign Funded Area Projects.
60. International Initiatives on Health Services/Systems Research.
61. Evolution of the Alma-Ata Declaration on Primary Health Care
and the Invention of "Selective Primary Health Care".
PART XII
POLITICAL DIMENSIONS OF HEALTH AND HEALTH SERVICES
62. Some Conceptual Issues About Intersectoral Development in
Health.
63. The National Health Policy of 1982 and After.
64. Political Economy cf Public Health Practice in India.
65. Politics of the Breakdown of the Public Health System.
PART XIII
METHODOLOGICAL CONSIDERATIONS FOR FORMULATING
RECOMMENDATIONS FOR ACTION TO DEAL WITH THE MALADIES
66. Developing Research Approaches by Conceptualising the Public
Health Services of the Country as a System.
67. Using Approaches of Operational Research and Systems Analysis
for Analysing Public Health Services.
68. Relevance of Going Back to India's Public Health Heritage to
Identify Remedies for Present Ailments of the Public Health Serv
ice System.
69. Emergence of Concepts and Methods of a "New Public Health".
PART XIV
A PRESCRIPTION FOR THE MALADIES
70. The First Steps in the Rejuvenation of the Public Health
Services of the Country.
71. Strengthening the Social and Cultural Foundations of the
Public Health Services.72. A Framework for a Health Policy in the New Pattern of
Panchayati Raj Administration.
73. Optimising Health Service Systems and their Different Compon- ■
ents.
74. Research and Action for Strengthening Health Manpower Devel
opment .
75. Developing a More Appropriate Cadre Structure for the Public
Health Services.
76. Towards an Endogenously Developed Body of Knowledge of Health
Economics for India and other developing Countries.
77. Developing an Alternative Framework for Dealing with AIDS as a
Public Health Problem by Adopting "Systems Thinking".
78. Enforcing Ethical Standards through the Medical Council of
India.
79. Policy Issues in the Promotion of the Endogenously Developed
Folk Medical Practices and the Indigenous Systems of Medicine and
Homeopathy.
83. Intersectoral Action to Improve the Health Status.
PART XV
81. Executive Summary.
PREFACE
Compared to the political leadership of the public health services that is
being provided these days (Chapter 64), that provided during the first two
decades after Independence appears as a Golden Age. Adapting the famous quota
tion from Moa Dzedong, "Politics was in Command" during those heady days.
Indeed, those two decades can be called as the Golden Age of Public Health in
India. This was in spite of the almost overwhelming problems the country faced
at the time of Independence. There was the lacerating trauma following the
partition of the country; there were the enormous public health problems; and
there was the acute dearth of resources for coping with the problems. The
momentum generated by the freedom struggle was still strong. The political
leadership had the courage of conviction to have a vision of the public health
services of the country. They dared to dream of making health services acces
sible to the huge masses of the people of the country who had been left out
till then.
Following this conviction, they had started to set up Primary Health
Centres (PHC) for,providing integrated preventive, promotive, curative and
rehabilitative health services to rural populations as a part of the overall
Community Development Programme. They had even echoed the recommendation of
the famous Bhore Committee that the services ought to be provided free cf
cost. There was the action to bring about social orientation of education and
training of physicians and other categories of health workers. The research
studies which culminated in the formulation of India's National Tuberculosis
Programme is still considered as a model for dealing with public health
problems all over the world. These not only provided the framework for FHO's
policy in this field, but three of the research studies - namely that heme
treatment is as good as treatment in sanatoria, that the BCG vaccine does not
provide protection at least to adults and that primacy ought to be given for
diagnosis and treatment to those cases of tuberculosis who are actively seek
ing help in different health institutions in a community - have radically
altered tuberculosis programmes even in the affluent countries. The National
Institute of Health Administration and Education was set up in 1964 to train
senior health administrators and to conduct research to strengthen health
administration of the country.
The political leadership was ably assisted in their efforts by a dedicat
ed and competent band of health administrators, most of whom belonging to the
cadre of the Indian Medical Service (IMS).
The year 1967 marks a watershed in the history of public health in India.
The year followed two successive failures of the monsoon; there was dangerous
political instability; foreign powers were poised to make their kill in this
country; there was acute economic crisis which was used by the foreign agen
cies to force a massive devaluation of the rupee.
Vivisection of the Ministry of Health into two separate departments of
health and family planning was a part of the pound of flesh extracted by the
foreign powers. This was done to give primacy to the now thoroughly discredit
ed Malthusian target oriented family planning programme in which coercive
pressures of various forms were applied the vulnerable sections of the people
to compel them to get sterilised. Four consequences followed almost naturally
from adoption of this infamous policy of population control : (a) the bureau
crats, who still carried the old colonial temperament of using strong-arm
tactics on the people, were considered as natural choice for carrying out such
hatchet jobs; (b)considerable amount of resources were made available to the
1
family planning department to support their coercive activities; (c) there was
not only neglect of the health department, but quite a few of its key activi
ties were in fact transferred - indeed “hijacked" - to the family planning
department to strengthen its not so reputable activities; (d) sensing further
scope for expansion of their power, the bureaucracy even extended its tenta
cles even into the moth-eaten department of health and soon became bold enough
to nominate themselves to international gatherings and even got jobs in inter
national organisations, which needed public health competence and which were
hitherto "preserves" of health administrators.
Political morality had also taken a steep downward turn: the ministers, the
cabinet and the Parliament were mute spectators of the macabre drama. Indeed,
politicians became a part of the unholy nexus of bureaucrats and foreign
consultants. To add to the misery of the masses of the people, the IMS was
made to wither away by this time and key public health positions were filled
by physicians who were patently unsuitable for the jobs. During the past
decade and a half, international agencies, with powerful backing from some
affluent countries, have imposed a number of ill-conceived, technocentric
programmes on the people of the country and there has been a virtual stampede
among bureaucrats and health administrators to legitimize such unethical
activities in the hope of getting some reward from the rich agencies. Thus,
the period since 1967 can be termed as a period when " Bureaucracy and Inter
national Consultants in Command", with a few crumbs being thrown to some
"obliging" physicians, who give such unethical acts an aura of pseudo-scien
tific respectability. Secretiveness and ostrasization of "undesirable persons"
become the logical outcome such moves.
The expected consequences of such unpardonable neglect of the public health
services by the political leadership started appearing very soon. Within a few
years there was a significant increase in the number of reported outbreaks of
what had been euphemistically termed as cases of gastro-enteritis simply be
cause the authorities concerned could not or did not examine the stools of the
victims for cholera vibrio. These outbreaks have since increased rapidly, both
in number as well as in size and possibly in virulence over the past three
decades, with little response from the authorities, apart from keeping the
information system in a state of virtual paralysis, apparently to avoid embar
rassment. The dreaded disease, Kala-Azar made its reappearance in North Bihar|
in the early 1970s, and has spread rapidly since then. Similar is the case
with the outbreaks of Japanese Encephalitis. Then, there were numerous unin
vestigated outbreaks of diseases such as infective hepatitis, bacillary
dysentery, meningococcal meningitis and cerebral malaria from different parts
of the country. There has been what has been called a virtual "Epidemic of
Epidemics", with little, if any response from the public health authorities.
They, along with their political masters, seem to have decided to "forget" the
people who had to bear the brunt of the epidemics and instead showed concern
about increasing incidence of Alzheimer's Disease or heart diseases among the
small segment of the people who count.
The .outbreak of the epidemic of plague in 1994 in two of the most advanced
states of the country was in a sense the culmination of this long neglect.
Probably the refractory authorities would have managed to weather even this
storm had it not created problems for international trade and India's "image"
abroad. But, predictably, the reaction was more cosmetic than substantial. In
stead of going to root cause, the Government of India played the well tried
trick of setting up bureaucrat dominated committees. A "high powered" commit
tee of secretaries from the concerned ministries was formed "to look into the
problem" of environmental sanitation which led to the outbreak of plague.
There was another committee dominated by microbiologists and government fur.e-
tionaries to look into the causes of the epidemic. A third committee was later
set up under a diabetologist/endocrinologist, who occupies the key post of a
member of the Planning Commission, to look into some of the problem of the
health services.
It was in the midst of such a situation that the Voluntary Health Associa
tion of India spearheaded a people's initiative to launch an autonomous body,
called the Independent Commission on Health in India (ICHI) to (a) examine the
state of those who "live on the other side of the moon"- the "forgotten peo
ples" - and how accessible are the health services to them; (b) examine the
phenomenon of "epidemics of epidemics" as symptom of breakdown of the public
health services in the country; (c) examine the performance of key public
health institutions of the country in the context of the breakdown of the
public health services; and, (d) find a way out of the family planning im
passe.
I readily agreed when I was invited to be a member of the ICHI, because
I share the concern of the convener. Soon, however, I felt that considering
line of work I have been following for over four decades, I would be in a
position to contribute to the issues raised by the convener of ICHI better if
I carried out an independent Project with the title: India's Forgotten Peoples
and the Breakdown of the Public Health Services - A Prescription for the
Malady. This was agreed to and the Project was launched by the Nucleus for
Health Policies and Programmes with soipe financial support from ICHI.
I had agreed to take up the Project because it falls within the central
interest of the work I have been carrying out throughout my life. As I have
mentioned elsewhere (Chapter2), the work on this Project covers a major por
tion of my intellectual autobiography. In carrying out this Project I have
brought to bear contributions from a very wide range of the work I have been
doing since 1950, when, while still as a student in the Medical College,
Calcutta, I took the momentous decision of setting my face away from the
traditional medical specialities and instead chose to work towards subordinat
ing medical technology to the interest of the people, particularly to the
unserved and the underserved. I have actually welcomed some constraints of
time which had to be imposed to synchronise it with the working of the ICHI
(about eight months) and also constraints of resources of different kinds,
because these will keep me within some boundaries, inside which I could take
full liberty to come out with my contributions to the subject of the Project.
The three major elements of the Project - the forgotten peoples, the public
health service system and coming out with recommendations for action - are
organically linked and they provide virtually endless scope to put across my
viewpoints. I have used a simile from clinical medicine to work on the last
two elements of the Project which concern the public health services - anato
my, physiology, etiology, pathogenesis, signs and symptoms, diagnosis and,
finally, management of the case. The conduits’ that I had developed to make my
presentation on the subject had opened up such a torrent of materials'that
have accumulated in my mind over the years that I have ended up writing as
many as eighty-one chapters for this work. There is no question of making any
claim that these eighty-one chapters cover the area comprehensively. That
simply could not be done, not only because of the most welcome constraints,
but also because the°range is virtually endless, both in extent as well as in
depth. This, again, was fortuitous, because it impelled me to "optimise" all”
that had to say on this virtually endless area within the constraints.
I make no apologies for taking the side of the people - Lok Paksh. Indeed,
it was the opportunity to put across the case of the forgotten peoples - to
bring back the memory of the state of those who are sought to be forgotten by
the political and bureaucratic authorities, often with full support from
foreign agencies which had impelled me to undertake this challenging assign
ment at this stage(66) of ray life. It was, however, kept in mind that the
cause of the forgotten peoples would be served better if their case is made on
the basis sound scientific data.
As described in detail in Chapter 2, I had made vigorous efforts to get
together the additional data that would be needed to cover the enormous area
that has been demarcated in the eighty-one chapters within the constraints of
time and resources. Obviously, it was simply unthinkable to have all the data
that I would have liked to have for all the chapters. I had to "optimise" my
efforts at data collection within the constraints. Roughly, I will venture to
estimate that I have attempted to obtain about twenty-five percent of the data
that_I considered important; the assumption here is that this twenty-five
percent constitute "the most important among the important". So frustrating is
the task of collecting reasonably sound data on the different aspects that I
would not have had the will to collect the remaining information and analyse
and interpret them for integration into the main theme of my presentation,
even if I was given the considerable more time and resources needed to do so.
If at all I had additional time and resources, and I could have mobilized the
energy needed for further work, I would have used that to further refine my
analysis, interpretation and conclusions and confined my efforts at additional
data collection only to some critical areas which are identified in the course
of the analysis. I am quite conscious that there is considerable scope more
work in presenting my ideas. However, I consciously chose to open up as much
area for analysis as I could at the cost of refinement of presentation. I
wouId,however, think that even with the information I have been able to col
lect, it has been possible to arrive at a diagnosis that is precise enough to
develop a dependable strategy for treating the malady.I will leave it to the
younger generation of concerned public health scholars to develop more compre
hensive, and if they so felt, an alternative thinking on the issues involved
to making the public health services more meaningful to India's forgotten
peoples.
Chapter 1 defines the research problem, which sets the stage for the study,
starting with the formulation of the research methodology, which is covered in
Chapter 2. Considering the enormous range of the research problem, the method
ology developed for the study had necessarily to be unique to conform to
complexity of the problem.
The following eight, chapters deal with the enormous problem of describing
and analyzing the forgotten peoples of a vast and variegated country like
India. Chapter 3 is focussed on issues concerning the political economy of
India's forgotten peoples. The work "peoples" (plural) is used frequently to
underline distinctive socio-cultural and geographical groups. The heading for
Chapter 4 is taken from a part of a quotation from Milan Kundera, who had
observed: "Man's struggle against oppression is a struggle between memory and
forgetfulness". This not only provides a backdrop for discussing the formation
of the sociology of knowledge to wish away the existence of the forgotten
peoples, but it also has helped in "reminding" the authorities about the past
achievements of the country in the field of public health and such remembering
can be of help in finding ways to improve the situation. Incidentally, another
term that has been used frequently in this chapter and elsewhere is "Marie
Antoinette Syndrome". This term is particularly apt to expose the hypocrisy of
the health economists, particularly the Indian ones, who echo Marie Antoinette
to legitimise economic liberalisation dictum of the IMF/World Bank :‘’no free
lunch", when it comes to the poor and therefore forgotten. Chapter 4 deals
with quantitative dimensions of the forgotten peoples. This required consider
able data from the National Sample Survey Organisation/Planning Commission,
4
but also to define who should be included to be termed as forgotten peoples.
Some space has been devoted
drawing up portraits of some families of "very"
forgotten peoples in Chapter
to remind the statistically oriented computer
loving mathematicians of what it means to left out by the uncaring society,
which swears by Marie Antoinette.
Chapter 7 contains some tentative thinking about the ecological consequenc
es of rising population in the country over the past forty-five years and how
they affect the forgotten peoples, how the country has managed to "absorb" an
additional 560 million people - more than 160 percent over the population in
1951; this puts the Malthusisns up-side down.. Data have been mobilised to
present a broad picture of the environmental conditions under which the for
gotten people are compelled to live and work in Chapter 8. To make up for the
virtual absence of key ep ider. io logical date , data produced in the previous
four chapters have been usee to provide a profile of the health problems faced
by the forgotten in Chapter 9.
Chapter 10 links the earlier seven chapters with parts of following chapt
ers as it presents the interface between people on one side and the public
health service system on the other. Contrary to what western trained health
educators have been claiming for quite some time, people do not to be educated
to seek treatment for their ailments. Education has to be directed to those
who have commodified practice of curative services and added to that profiteer
from the. sufferings of the people.Failure of the public health services to
meet the (felt) needs of the people creates "market" for the much admired
private sector doctors, both qualified and otherwise, who follow the rule of
the jungle to exploit the suffering of the people by taking recourse to pat
ently unethical practices (see Chapter 53).
Chapter 11 contains clarification of the meaning of public health, because
considerable confusion has been caused because of its misuse by those who have
not paid adequate attention to its concepts, methods and even definition. This
was done to provide a framewrrk for discussion for subsequent chapters. That
is one reason the term "public health services", rather than "health services"
has been mostly used, even though they carry the same meaning. Before embark
ing on more detailed analysis.description, an overview of the “sickness" of
the public health services cf the country is presented in Chapter 12 to give
an abstract of the current state of affairs. Chapter 13 follows it up with
data from two nationwide sample surveys ostensibly to assess the pattern of
utilisation of curative services. Although both these surveys present similar
pictures, inadequacies in the understanding of the structure and function of
the public health system of the country and a lack of appreciation of the
interrelation between cultural perception of health problems, access to insti
tutions providing health services and cultural meanings and perception of
health problems, .. as discussed in Chapter 12 and elsewhere, could have
avoided some problems in the conceptualisation of the research
problems and in the survey designs. Despite these limitation,
however, these two survey have given most valuable nationwide data
on -behavior of the people (under the existing state of the ..public
health services) when they get sick.
Chapter 14 recounts the terror caused among health workers and
target oriented
the vulnerable groups of the population by the
family planning programme for more than three decades. It has
caused extensive
damage to many cf the facets of the public
health system of the- country. It has been described as furious
bull in the china shop of th«- health services. Chapter 15 gi s a
very brief account of another nationwide population survey - the
National Family Health Survey. This is confined to contraception
behavior of mothers and many other what is peculiarly described as
"family planning related health activities" - it is the tail
wagging the dog. This, incidentally, is a major pathological
feature of the health services of the country. Chapter 15 de
scribe ICMR studies of "family planning" and "family planning
aspects of health activities" in many of the PHCs. Although many
serious limitations have been observed in the two research stud
ies analyzed here, this marks the beginning of analysis of func
tioning of PHCs. Women's health and health services issues have
been taken up in Chapter.17'to underline the importance of linking
these issues with the overall strategy of development of the
public health services, and to consciously avoid the trap of
starting a separate outfit to look after women's health problems.
The crucial analysis of the state of the PHCs in the country is
presented in Chapter 18, where the fragments of information ob
tained through data in Chapters 13, 15 and 16 are complemented
with more holistic analyses of the PHCs in different parts of the
country’, including direct observations of their functioning in the
states of Kerala and Tamil Nadu. This chapter forms the beginnings
of the account of the grim state of the health services in the
country. The confusion concerning Community Health Centres and the
New PHCs is widespread. It is a reflection of the utter confusion
that prevails at the Department of Family Welfare of the Union
Ministry of Health and Family Welfare and in the Health and
Family Welfare Division of the Planning Commission, who had ini
tiated this ill-conceived scheme in the first place. A twentythree year long account of the eleven PHCs (1972-95), which were
included in the Nineteen Village Study and which included PHCs
from West Bengal, Kerala, Tamil Nadu, Karnataka and Gujarat, give
Ln Chapter 19, fitted in very well with Chapter 18 to give de
tailed "flesh and blood" account of the working of PHCs over a
long span of time. The picture that emerged from the data present
ed in this chapter gave a graphic description of the devastating
consequences of the of the irresponsible policies and programme
imposed by the Union Ministry, often at behest of the foreign
agencies. It also showed how passively the states abdicated their
constitutional prerogative of formulating their own health po
licies and programmes and succumbed to the money dangled before
their eyes by the Union Government as quid pro quo for following
the "party line". It is ironical that documents on the health
services produced by the Subjects Committee of the West Bengal
Legislative Assembly repeatedly refers to "central directives".
How can a state, and a Left Front controlled state at that, become
subservient followers of instructions from some ill-qualified
joint secretary from the Union Ministry of Health and Family Plan
ning? The IAS state health secretary knows full well • which side
his/her carrier interest lay and the Marxist state health minister
"allows" the secretary to follow the party line dictated by the
centre!
The PHCs were virtually non-existent in the areas inhabited by
some of the particularly disadvantaged groups among the forgotten
peoples, namely the Adivasis, the desert people and residents in
remote hill areas. Added to that their cultural characteristics
and special ecological conditions of living called for something
different from the stereotyped "central pattern" that is dished
discussed in Chapter 28 and the National AIDS Control Programme
has been taken up in Chapter 29. The claims concerning "elimina
tion" of leprosy form India by the turn of the century has been
examined in Chapter 30. Chapter 31 deals with the National Blind
ness Control Programme. It examine how appropriate it is to con
fine the focus on treatment of cataract for "prevention" of blind
ness in the country. Reference has been made earlier to the out
break of an epidemic of epidemics as a result of long neglect of
certain basic public health services in the country. This aspect
is analyzed in Chapter 32. Analysis of national programmes ends
with a discussion on two contrasting components of nutrition
programmes - the Integrated Child Development Scheme and the
national programme for eradication of iodine deficiency diseases.
Chapter 33 is devoted to this area.
Analysis of the "health care delivery system" and th? interface
between this and the people, particularly with the forgotten
peoples, made in Chapters 11-33, is followed with an examination
of the way the public health services are being administered in
the country. The entire system of health administration, from the
top level to the bottom, is considered in Chapter 34. This chapter
gives an account of the processes which have led to the decline of
the quality of practice of public health services in the country.
An entire speciality, which is so critical for effectively running
the public health services of the country, has been "forgotten" to
give a free play to narrow interests of some people who happened
to have acquired positions of authority. Obviously, "remembering"
or retrieving the forgotten principles of health administration
will be a major piank for recommending ways rejuvenating the
public health services of the country; the forces which led to the
“forgetting" health administration as a speciality will have to be
countered, if the decline is to be stopped and reconstruction of
the public health services is to be begun. The degree to which
bureaucrats have infiltrated into the decision making process in
public health, which requires special competence, is the subject
of analysis in Chapter 35.
Health manpower development (now often termed.as "human re
sources for health") is another important area which needs to be
analyzed as one of the components of the public health system of
the country. While planning this Project, it was estimated that it
could be discussed in a single chapter. However, as the thinking
proceeded in this area, it soon became clear that even with the
limited discussion which will serve the purpose, as many as nine
distinct areas will be delineated to cover this field. These all
needed separate treatment in individual chapters. The "sub-area"
of medical education and the dynamics of the medical profession
klone needed five distinct chapters. Considering the fact that a
number of socio-politicaJ. forces are at play in shaping both the
quality and the extent c£ medical education since independence,
the latest being the mushrooming of the of the so-called capita
tion fee medical college, a separate chapter, Chapter 36, was set
aside for this field. The long account of the gradual decay of the
quality of medical education, which was . almost an inevitable
consequence of the interplay of the socio-political forces, is
covered in Chapter37. Adequate material was brought together on
the subject of the role of those who were expected to serve as
guardians of medical education in the country to warren a separate
discussion on it in Chapter 38. How these and other socio-economic
changes have brought about changes in the medical profession over
the decades since Independence is discussed in Chapter 39. Consid
ering very serious ethical issues that have emerged as result
commodification of medical practice and conscious steps taken by
the government to continue tc expand the private sector in cura
tive medicine, the trend of "Machiavelli exerting pressure to ease
out Hippocrates" has been growing in strength. This aspect is
dealt with in Chapter 40. Thus, it can be seen that increasing the
number of chapters was quite desirable for getting some of the key
ideas on this important companents into sharper focus.
The insights provided through a study of the sociology of the
formation of the nursing profession in the context of the change
in the cultural and religious setting in the country and evolution
of education and training cf various categories of nurses has
important bearing on the partem of manpower development. This
aspect forms the content of Chapter 41. Chapter 42 contains dis
cussion on some of the other health professionals who should be
considered for health manpower development. Another important
element of health manpower is formed by the so-called paramedical
personnel, who have played such a crucial role in development of
the public health services in the past. This is discussed in
Chapter43. Chapter 44 deals with the research basis for health
manpower development in the country. This forms the sheet anchor;
it is the pattern of the public health services, which is evolved
through health.systems research, which determines all the three
aspects of health manpower development - planning, production and
management of the manpower ne-eded for the public health services.
Manpower development ought thus follow health systems research to
optimise health service systems.
A number of key health institutions have been set up to pro
vide support for the health services in the form of education,
training and research in public health. Again, it was intended to
discuss their activities in -he context of the existing state of
the health services in the country in a single chapter. However,
it was felt that the purpose will be better served if five these
institutions are very briefly described and analyzed in four sepa
rate chapters after presenting an overview of the degree to which
these key institutions have deteriorated over the years in Chapt
ered. The most disturbing finding in this chapter is the extent to
which the authorities have managed to “influence" these key insti
tutions to toe the line laid down by them. Expectedly, many of
these institutions were mobilised to provided "academic" support
to the much discredited family planning programme of the govern
ment. The government interference in the working of these insti
tutes has thus been a serious factor to account for the decline of
these institutions. Despite allocating separate chapters for three
big institutions and another to discuss together another two of
them which are directly under the control of the government, only
very limited aspects of these institutions are taken up for the
purpose of this study. The Indian Council of Medical Research,
which is required to play s pivotal role in promoting medical
research of various types, i.u dealt with in Chapter 46. The All
India Institute of Medical S:. ences, which was required to provide
research support to medical education and train teachers for
undergraduate and postgraduate education in the country as a whole
is analyzed in Chapter 47. The decline of the National Institute
of Health Administration and Education, which was later "hijacked"
by the family planning department after it was merged with the
Central/National Institute of Family Planning to form the present
National Institute of health and Family Welfare, is discussed in
Chapter 48. The deplorable conditions of the All India Institute
of Hygiene and Public Health, which played a pivotal role in the
development public health services in the country, including the
formation of the recommendations of the Bhore Committee and of the
National Institute of Communicable Diseases, which set up to
perform functions similar to the ones assigned to the famous
Communicable Diseases Center at Atlanta, Georgia, USA, are dis
cussed together in Chapter 49. These discussions have formed the
bases of some of the recommendations for rectifying the situation
obtaining today.
Another component of the public health system of the country is
the pronounced regional variations which have been pointed out in
different chapters. An extreme instance is found in the case ofthe Adivasis and the people who live in desert and remote hill
areas. This subject is essentially a political, economic and
ecological question. Some of the ideas on this aspect is brought
together in Chapter 50.
The next three chapters deal with some more recent factors etiological . factors - which have exacerbated the already serious
sickness of the public health service system of the country.These
have proved to be some sort of a virus, or, more appropriately,
iatrogenic agents for the system. The World Bank brand of health
economics, which was avidly endorsed by some high profile econo
mists of the country, has turned out tc be a virulent form of a—
iatrogenic agent. It is called iatrogenic because it diverts
attention from the core problems and it simply ignored some other
critical interdisciplinary dimensions that form parts of the
process of health policy formulation. The central concept of the
World Bank economists, namely calculation of cost-effectiveness of
alternative health programmes in terms of "Disability Averted Life
Years" (DALY) saved, is utterly indefensible as a research tool.
More recently (December 30 1995), the Harvard economist, Amartya
Sen has
also raised doubts about this approach by bringing u? the
question of equity, which happens to be a major policy guideline
for the World Bank economists and their many Indian admirers. Some
ethical aspects of practice of this type of public health quackery
are discussed
in Chapter 51. The far reaching consequences of
slashing of health budgets at the centre and in the states in the
■ wake of implementation of the structural adjustment programme
prescribed by the World Bank/IMF are examined in Chapter 52. Th®
associated unleashing of the private sector, which has exploited
the poorly regulatory conditions to steep to most unethical medi
cal practices to make profit from the suffering of the people of
the country, is discussed in Chapter 52.
The next three chapters deal with seme associated factors whicn
contribute to the system. The bold recommendations on national
drug policy by the Hathi Committee has been consistently watered
down by the authorities. Expectedly, the new economic policy hass
. IE
added to this trend of wiping out the very modest concessions that
had been wrested by the poor in the wake of the Hathi Committee
recommendations. This aspect is dealt with in Chapter 53. The
focus of Chapter 54 is on the politically motivated approach to
the issue of use of the indigenous systems of medicine in the
public health system and how this has distorted the intrinsic
value of integrating people's own coping mechanisms with health
services that are made accessible to them by the authorities.
Chapter 55 contains an account of the very sharp changes that have
occurred in the work of the voluntary sector, now taking on .the
more apt label of non-governmental organisation, a substantial
majority of them almost openly serving as conduits for penetration
of the prefabricated programmes of foreign agencies and other
vested interests.
The following five chapters deal with yet another type of
iatrogesis in the form of involvement of foreign agencies in
India's public health programmes. The World Bank looms largest
among them. Then there are other international organisations like
the WHO and UNICEF. There also individual countries and the big
foundations associated with them and a large number of non-govern
mental agencies providing funds for their Indian counterparts.
This "multi pronged" moves pose a real problem the system. Chapter
57 discusses the metamorphosis that has occurred within WHO and
UNICEF, when they got actively involved with programmes which go
contrary to what they themselves Jointly helped to articulate in
the Alma-Ata Declaration. An account of some of the programmes
that have been imposed on the country by some international organ
isations, ignoring studies which had seriously called into ques
tion their scientific validity, is given in Chapter 58. Seme
research programmes launched by a number of foreign agencies,
headed by the World Bank with its India Population Projects, are
critically analyzed in Chapter 59. The failure of the initiative
taken by the much publicised International Commission on Health
Research to promote worldwide what it had termed as Essential
National Health Research, has been documented in Chapter 60 in the
form of analysis of the follow-up action during the subsequent
three years. Chapter 61 sums up the activities of some interna
tional and foreign agencies in the form of presentation of evolu
tion of the Alma-Ata Declaration and how the western countries
have managed to make the countries of the world "forget" about it
by inventing what it euphemistically called Selective Primary
Health Care.
The next four chapters are related to political dimensions of
development of the public health services in the country. Chapter
62 deals with the question of developments in many fields other
than the public health services which influence the state of
health of a population. The political forces which were instrumen
tal in the enunciation of the National Health Policy of 1982 and
the factors which came in the way of its implementation are dis
cussed in Chapter 63. The political leadership has represented the
forces which have actively tried to wish away or "forget" the poor
and the downtrodden peoples of the country and which has allowed
the public health services to deteriorate to such an extent.
Chapter 64 cites some facets of the "politics" of the breakdown cf
the public health services of the country. The political economy
of the health services of the country is discussed in Chapter 65.
The remaining fifteen chapters deal with process of formation
of the recommendations on the basis of the diagnosis arrived at in
the course of elaborations in the previous fifty-five chapters and
on the recommendations themselves. Chapter 66 deals with the
central theme of the formation of the recommendations - developing
research approaches by conceptualizing the public health services
as a complex, interacting system. Another of the methodological
approaches which has been frequently been used in India, namely
the use of operational research and systems analysis, is discussed
in Chapter 67. We in this country are fortunate that we can go
back to - "remember" - our past experience in conducting health
systems research to lay the foundations of India's health services
in order to find a way out of the present crisis. This has been
recalled in Chapter 68. Chapter 69 sums up the new conceptual ap
proaches that have been used in the formulation of the recommenda
tions for action.
With the methodological background given in Chapters 66-69,
taking account of the enormous data which have led to the "diagno
sis" of the malady, the main "prescription" for the malady of the
public health services of the country is given in the next eleven
chapters. These chapters are presented in terms of gradation of
their importance and in terms of a broad sequence that has to be
followed in “managing" the patient. Chapter 70 contains by far the
most critical component of the prescription. This, therefore,
occupies the highest grade and forms the pace-setter for the
subsequent recommendations for action. As the failure of the
political leadership to safeguard the interests of the community
is held as the most important etiological agent in the causation
of the sickness of the public health system, the pace for the cure
has to be set at that level.lt is contended that if that is not
done, then it will be unreasonable to expect much improvement .The
other eight steps that are to’ follow a political commitment are :
revitalization of the key public health institutions; placing
qualified and competent persons at key public health posts; roll
ing back the bureaucrats from the positions for which they have no
competence; restructuring the family planning programme; integrat
ing the vertical programmes with the general health services;
insisting on a position of "equality" while interacting with
foreign agencies; rectifying the regional imbalances in health
service development; making available funds once these changes are
effected.
A set of three chapters, which deal with some basic principles
of development of the health services, form the next gradation.
Chapter 71 deals with the steps that need to be taken to relate
health services to the people, particularly with the forgotten
peoples. Chapter 72 discusses the opportunities provided in the
moves to decentralize administration through constitutional provi
sions offer valuable possibilities for decentralisation of admin
istration of the public health services. Chapter 73 contains the
principles for developing programmes which ensure optimum use of
the available resources.
The last seven chapters give more specific examples for improv
ing the system, once the basic requirements for initiating actions
are met. They thus form the next grade of the prescription. The
important question of steps to be taken to strengthen health
manpower development to meet the challenge of developing a more
effective public health service system is discussed in Chapter 74.
Chapter 75 deals with another important area of restructuring the
health service cadre for personnel to conform to the new require
ments. In view of the many flaws identified in the World Bank
approach to health economics, an outline of an endogenously devel
oped alternative is given in Chapter 76. The example of the Na
tional AIDS Control Programme is taken in Chapter 77 to elaborate
how even an effort to develop a "system thinking" can be of help
in articulating better alternatives. Chapter 78 focuses on the
steps to be taken to strengthen the working of the Medical Council
of India so that it more effectively deal with the critical prob
lems of improving the ethical standards, the quality of medical
education and regulate the quality of medical practice. Chapter 79
contains an alternative approach to integrate the practice of folk
medical practices and practice of the indigenous systems of medi
cine and homeopathy within the public health services. The presen
tation ends with the Chapter 80 which underlines the importance of
improving the other facets of the lives of the forgotten peoples
for improving their health status.
An executive summary of the contents of the eighty chapters is
given in ChapterSl.
It simply "happened"; as set about to think of the content of
the project, ideas came gushing in rapidly and I had to go or
assigning separate chapters as they got articulated to take forms
of individual identities. After having all the birth pangs of
creativity, it feels good that I managed to put together r.y views
in the eighty-one chapters.
A technical mishap, which occurred recently, is being mentioned
here as it provides an interesting insight into the precess of
writing this voluminous monograph. When I finished writing the
"first" Preface for this monograph in the middle of December 1995,
something happened in my personal computer due to which the entire
work got "corrupted" and the computer doctor pronounced that it
got almost irretrievably lost. It was shattering blow, because I
did not have any notes and I was so exhausted
both mentally and physically that I simply did not have the energy
to reconstruct the preface. However, after a week's a state of
mental disorientation, I started to pick up the threads to recon
struct in the form the present Preface. When I was reaching the
end of this second effort, the good computer doctor rushed in to
announce the success of his operation to retrieve the old preface.
This gave me a chance to compare the two versions it was found
that while I had been surprisingly successful in retaining the
core of the ideas of the first draft in the second (ie. the present'one), the two differed considerably in "flavor". This gave me
an idea of what would have happened if due some misfortune, I had
lost all the eighty-one chapters and forced by some miracle to re
write them! In that hypothetical situation, the entire book would
have carried a different "flavour" than what is presented in this
monograph, though, the "core" would have remained substantially
unchanged.
I was very disappointed that once again T had to plough a
lonely furrow in carrying out the Project. I had sent personal
letters and a copy of the Project proposal to more than thirty
scholars who have great interest in this field, inviting them to
offer their comments and criticism and to join me in the work in
parts in the whole. This included personnel working with the World
Bank, UNICEF and WHO.The disappointment was particularly great in
the response FROM the younger generation. Expecting greater enthu
siasm from them, I had proposed to have a two to three days'
"brain storming" session with groups of interested scholars in
Delhi, Bombay and in Bangalore on a discussion paper specially
prepared by me. I drew a blank from all the three groups. I re
ceived comments from Rajni Kothari, V Ramalingaswami, Uton Muchtar
Rapphei (from WHO/SEARO) and my friend, J H Deisfield from the
University of Heidelberg. All of them wrote to encourage me in my
venture; there was also an undercurrent of concern whether I had
not taken too exhaustive a work within the limited time frame. I
express my thanks to them.
I also record my thanks to C K Jagadeesan, who as research
assistant gave me most valuable support in the locating the needed
documents, in accompanying me to the fieldwork in Kerala and Tamil
Nadu and in taking care of the many logistic problems. M D Rastogi of the Documentation Centre of the Centre of Social Medicine
and Community Health of Jawaharlal Nehru University was cf great
assistance in providing documentation support.
DEBABAR BANERJI
Nucleus for Health Policies and Programmes,
and
The Centre of Social Medicine and Community Health,
Jawaharlal Nehru University,
NEW DELHI.
January 1 1996:
Mailing address: B-43, Panchsheel Enclave, NEW DELHI 110017.
VOLUNTARY HEALTH ASSOCIATION OE INDIA
40, INSTITUTIONAL AREA.
Phones : 668071, 668072, 665018
SOUTH OF I.I.T.,
NEW DELHI - 110016
Grams : “VOLHEALTH" New Delhi-110016
The ©rag Kssrae amdl the National Drag Policy:
Health, Development and Politics
Mira Stsiva
India’s National Drug Policy is in the offing. While different
sectors in the drug industry are busy lobbying for concessions and
greater profit margins, the voice of the consumer is conspicnous by its
absence. Unused to participating in decision making or even analys
ing and expressing their views regarding health and drug policies, the
academic and professional medical bodies and faculties of medical
colleges and the health professionals have very little to contribute.
Hence, the medical establishment is not in a position to safeguard the
health of the people, or even meet their real health needs. Expecting
it to fight on behalf of the people for a rational health and drug
policy is being unrealistic.
More tragic has been the non-role of the social action groups,
many of whom have tended to look upon health work as a ‘relief’
and ‘reformatory’ activity, and health issues as non-political. Trade
unions, while being concerned about wages and bonuses, have not
cared to prevent the exploitation of their workers and their meagre
resources by the increasing commercialisation and pharmaceuticalisation of health care. One would have expected protest against a drug
policy which is not in the interest of the people to come from some
such groups as also expect their support for such a protest made by
others.
It is crucial that people in all walks of life understand the drug
situation in the country, the implications of the policy changes being
formulated and their impact on their own life and that of other
people.
Need of a rational drug policy
A Drug Policy is an integral part of a health policy. The increas
ing dependence on drugs and medical technologies has successfully
Dr Mira Shiva works with the Voluntary Health Association of India.
New Delhi as Co-ordinator of Low Cost Drugs and Rational Therapeutics and
is Convener of the All India Drug Action Network.
1
managed to create 'myths’ aad 'beliefs’ that there are medical
solutions to problems arising out of unmet basic needs, solutions for
which can only come from socio-economic and political changes. With
the proliferation of health personnel (trained and untrained) chemist
and druggist shops even in remote rural areas, the ‘pill culture’has
infiltrated wide areas and communities through ‘commercial’ channels.
Misguided yet genuine efforts of development workers to ensure that
the poor villagers get a share in the fruits of‘modern science’have
converted millions into consumers with artificial drug needs. This has
created profitable markets for the drug industry. Unfortunately, the
controls that should have gone with this development, such as the
availability of unbiased drug information, the warnings etc., are
woefully missing.
Increasing dependence on drugs is not merely marginalising
and systematically eroding the previously existing alternatives, many
of which were holistic, low cost, locally available, indigenous and en
couraged self-reliance, but most of the drug consumption is for self
limiting trivial health problems. Local alternatives for these have
existed and are still often the most appropriate and safe. For people’s
health rights to become real, a drug policy has to remove this artificial
dependence. A drug policy can complement health care efforts or
totally cripple them. We can choose an unhealthy dependence on the
medical-industrial complex in spite of its very little impact on th®
health status of our people or we can ensure a rational use of drugs
which is in the best interest of the people.
WHO’s Action Programme for Essential Drugs has drawn up
guidelines for rational Drug Policies, which are therapeutically,
medically sound and economically speaking the most appropriate.
While most WHO programmes are given whole-hearted support,
where WHO’s Action Programme for Essential Drugs is concerned,
vested interests have blocked it from, being given the support it
deserves. Thus the drug industries seem to not merely dictate (subtly
brainwash) the prescription practices but also influence the drug
policies themselves. This indicates their increasing power and our
continuing lack of strong reaction.
India has one of the best developed pharmaceutical industries
in the Third World. According to UXIDO (Lisbon Conference),
India’s pharmaceutical industry was considered to belong to category
V, i.e. technologically developed enough to be totally self-reliant. Yet
many ills ail the pharmaceutical scene and some of the ills are critical.
India’s Pharmaceutical growth has been very impressive:
2
Table 1: India’s Pharmaceutical growth 1952-53 to 1982-83
SI. No.
Item
I.
2.
3.
Number of units
Investment
Bulk drug production
4.
5.
Formulation
Imports
6.
7.
8.
Exports
Price index of drugs
Price index of all
commodities
1953-54
1982-83
% increase
1643
Rs 24 crore
Rs 27 crore
(64-65)
Rs 35 crore
Rs 16 crore
(51-52)
Rs 0.8 crore
100 (1970-71)
100 (1970-71)
6631
Rs 600 crore
Rs 325 crore
300
2,400
1.800
Rs 1545 crore
Rs 141 crore
4,300
780
Rs 111 crore
167
287
13,700
67
187
Source: Gharpure 1985: 37.
In spite of this increase, drug production, distribution etc.
have not been in keeping with the health needs of our people. While
on the one hand shortages of essential and life saving drugs have
occurred, on the other even rural markets have been flooded with
costly brands of absolutely irrational drugs of doubtful therapeutic
drug needs.
Disparities between needs and production
For the majority suffering from ill health, their health problem
stems from unmet basic needs of adequate food, safe drinking water
and healthy working and living conditions. For the right to health to
be real, these issues have to be tackled. But in practice it does not
happen. As a result, the poor who have health problems because of
food deficiency (and low income) are ignored. While production and
availability of pulses has decreased, the so called high protein food
supplements like protein Complan, Bournvita are pushed vigourously
in the market (Gopalan 1985: 12-13). The average growth rate (height)
of rural children is far less than that of their urban counterparts.
There has been a deterioration in the growth of the poor Indian
children between 1957 and 1978. Food availability now is 10% lower
per capita, per year, than in 1953-54 (Gharpure 1985). According to
Dr. C. Gopalan (1985: 12-13), ‘only less than 3 million of the 23
million to be born in 1983 will become truly healthy, physically fit,
productive and intellectually capable citizens of this country.’ In
India we have over 30,000 children becoming blind each year (and
this is a gross under estimate) because of the failure to get vitamin A
in their diets (UNICEF 1984: 52). Children who are denied adequate
3
protein, calories, essential vitamins and minerals can in no way com
pensate for them by drinking costly tonics (Jayarao 1976).
Table 2: Targets and Production of Monitored Bulk Drugs
1980-81
target-prod
UNIT
Vit A MMU 66.0 59.8
1981-82
tar-prod
66.6 52.6
1982-83
tar-prod
77.0 52.49
1983-84
tar-prod
90 60.23
1984-85
tar-prod
105 55.9
Sources'. Narayana 1984: 244; Govt, of India 1985: 38.
With the increase in total population and with 42 per cent of
our population below the age of 15 and about 50 per cent below the
poverty line and malnourished, requirements of Vit A are definately
much more than the production target (Tabic 2). While a tremendous
gap exists between the target and the estimated production of Vit.A
drugs in 1984-85, the production itself has gone down from 62.2
MMU in 1978-79 and 59.8 MMU in 1980-81 to an estimated 55.9
MMU in 1984-85. Given the increase in requirements, this depreciation
is significant
India has over 60 million people with endemic goitre. Iodine
deficiency in pregnant women and children is known to be associated
with birth of deaf, mute or mentally subnormal children. Over 1,000
significantly mentally deranged children arc known to be born in the
Terai region alone. In areas with 50 per cent population with goitre,
2 per cent of all children born are expected to be abnormal (Gopalan
1985). All this is for want of iodized salt. We produce only 20 per cent
of our overall requirement of iodized salt, much of which does not
even reach the endemic areas (Nutrition Foundation of India 1983).
If such large numbers are allowed to be born underweight and
allowed to develop only to be blinded, maimed or mentally retarded—
with health and drug policies failing to change the situation signifi
cantly even after 38 years of independence—then the policies which
have already been found to be inappropriate need to be totally
revamped with relevant restructuring. The Government of India
(1982) itself has acknowledged that
the imported and inappropriate model of health services is
top heavy, over centralised, heavily curative in its approach,
urban and elite oriented, costly and dependency creating.
India has 10 million TB patients, out of which 2.5 million are
actively infectious and 50,000 die each year. We produce J of the
mininum requirement of anti-TB drugs, according to an 1CSSR-ICMR
Report. While the production rate of basic and cheaper anti-TB drugs
has tended to be inadequate, costly second line drugs have been
vigorously pushed. To cite some statistics, whereas the target for
production of IN drugs which are cheaper, had gone up marginally
from 140 tons in 1981-82 to 158 tons in 1982-83, the target for costly
drugs like Ethambutol had shot up from 32 to 154 tons.
Non-essential drugs
75 per cent of the drugs in the market are non-essential and 25
per cent of our drug market constitutes of tonics, vitamins, tonic
restoratives, enzymes etc. (ICSSR-ICMR 1981). Tonic sales in India
is 12 per cent as against 3 per cent in developed countries. On the
other hand, sale of Vit.A is a mere 3 per cent. In 1977, an UNCTAD
study showed that 34 per cent of all drugs sold were tonics, tranquil
lisers and cough mixtures. Anti-microbials were a mere 2 per cent and
essential vaccines merely 7 per cent. In 1980, the production of
vitamins, tonics and cough and cold remedies amounted to 23.5 per
cent alone.
The Hathi Committee (Government of India 1975) drew up a list
of 116 essential drugs for India. The World Health Organisation (1977)
recommended around 200 essential drugs. Sweden has 2,000 drugs
in the market. India has 30,000 formulations out of which 5,000 are
useful and 2,500 of marginal use (Gharpure 1985: 326). 68 per cent of
the drugs are classified as obsolete by the DCC. Of these 30 per cent are
useless and 70 per cent are actually harmful. The best private hospitals
in U-S. possess their own hospital formularies with restricted drug
lists. U.K. has recently decided to restrict the drug list in the National
Health Services at a considerable saving to the Nation (as a recent
issue of the Guardian (1985) has brought out).
WHO has given very clear guidelines regarding selection of
essential drugs. It is upto the different countries to draw up their
own lists based on their priority health needs, to guide drug produc
tion, drug distribution, drug information, quality control etc. Fiscal
reasons have guided the entire policy formulation in our country,
much to our regret (Melrose 1983).
While WHO’s essential drug list has been considered extremely
short and arbitrary by the drug industry as well as our own drugs
control authorities, slashing the essential drug list under price control
from 343 to 95 merely to ensure that the prices of all other drugs are
decontrolled, does not make sense.
Continued sales of banned, bannable and hazardous drugs
It is amazing, how with imports of ‘high-technology’ rarely are
the ‘controls’ and 'precautions’ imported. Technological know-how
has come to mean know-how required for putting up the production
plant. The knowledge of the status of the product in terms of
5
efficacy, safety, comparison with other drugs, evaluation studies in
other parts of the world are all part of the know-how. Numerous
unsafe and controversial drugs continue to be marketed. For exam
ple, while 1.5 million Indian children die of diarrhoea each year,
the drug market of anti-diarrhoeal is flourishing. Message of ORT
is drawn in the glib sales talk for anti-diarrhoeal. Lomotil, known to
have caused several infant deaths due to its extremely narrow safety
margin, continues to be given to children (Medawar and Freeze
1983).
Clioquinols—Hydroxyquinolines (Mexaform like drugs): The
crippling and blinding of over 11,000 Japanese with mexaformenterovioform is well known. Efforts by drug companies involved
and the medical community to associate SMON Syndrome (Subacute
Myelo Optic Neuropathy) with ‘virus’ ‘genetic’ causes were
countered by a group of socially conscious lawyers, journalists and
doctors. Dr Olle Hansson showed the association of blindness
(optic neuritis) with mexaform and enterovioform and proved tnat
the drugs were absorbed from the gut and therefore could affect the
nervous system. Efforts of Japanese lawyers and SMON victims to
get these drugs withdrawn worldwide, succeeded in forcing CibaGeigy to withdraw Enterovioform and Mexaform from the world
market by March 1985.
Over 2,000 Mexicans died in 1972 in a Typhoid epidemic
because resistance to chlosamphenicol had emerged unrecognised by
the medical professionals. Emergence of resistance to a drug means
failure of the drug to destroy the harmful organisms. Typhoid resis
tant to chlosamphenicol in endemic form is being reported from
Kerala and is spreading. Resistance to majority of the commonly
used antibiotics contributed to the deaths of over 2,000 in 1983 in the
West Bengal dysentry epidemic. Besides death and uncontrolled
spread of diseases, resistance due to misuse of patent antibiotics for
trivial problems means dependence on costly, unaffordable alterna
tives.
The Thalidomide disaster sums up the problem of hazardous
drugs and their implications. Over 16,000 children were born mal
formed, invalid for life because their mothers consumed Thalido
mide—a drug which was sold to them for morning sickness. Obvi
ously tests on effect on foetus was not done.
The E.P. drug story is a medical crime story. In 1976 WHO
based reports from Australian Medical Association warned all tue
member countries not to use high dose estrogen-progesterone drugs
for pregnancy testing—as it was found to be associated with foetal
malformation. But in India till 1982, drug marketing literature
6
f)
advocated the drugs for pregnancy testing. What was probably most
horrendous was the fact that not merely were these drugs being pre
scribed freely by the doctors without any warning to the mothers, who
had every intention of having their (healthy) babies, but these
products were sold freely over the counter without warning to any
one, for the asking. The drugs were consumed not merely for preg
nancy testing but also for inducing abortion and several other gyneco
logical problems.
Warnings in medical jargon, warnings in an alien language,
warnings in microscopic letters, even if written would not have made
any sense to the women. But not even these legalities are observed
in all these harmful drugs. Following a national campaign against
the drug and public pressure demanding a ban, the drug was banned
by the Drug Controller of India. But some pharmaceutical compa
nies went to court and got a stay order against the ban. Thus they
continue to produce these and other harmful drugs and do not even
give the statutary warning—even in an alien language.
Inadequate drug control
Five quality control laboratories and 500 drug inspectors attempt to
maintain quality control of drugs produced from over 8.000 formulators and sold by several thousand drug distributors or chemists in the
entire country. The recommended ratio of drug inspectors to drug
units is 1:25 and of drug outlets is 1:100, but the numbers are grossly
inadequate. Japan which is considered to be the seat of small scale
industry has only 1,000 drug units. U.S. with 16 per cent of the drug
market has only 700 units. India with a mere 1 per cent of the
world market and 30-40,000 formulations, has 8,000 formulators.
Quality control is bound to suffer and lead to sales of substandard
and spurious drugs. 20 per cent of the drugs in the market are sub
standard as accepted in the Parliament.
Complaints and demands for increase in the number of quality
control labs for drug inspectors has been evaded by statements from
the authorities complaining of ‘financial constraints.’
There is no doubt that enough legal loopholes exist. These
ensure that almost each and every case fought by the government
authorities against the drug houses who flaunt the drug legislation is
either painfully prolonged or lost. In 1980 the drug consultative
committee reviewed 34 categories of drug combinations of which 23
categories were recommended for being weeded out with 16 catego
ries to be removed immediately. In 1981 -.he Drug Advisory Board
diluted the list by excluding some of the drugs from the list.
7
In July 1983, the Drug Controller of India issued a gazette
notification banning 22 categories of drugs. In 1984, several of these
drugs were available in the market. And today (in 1985), many of
the banned drugs are still available. Many doctors and chemists are
not even aware of the ban. Failure on the part of the Drug Control
authorities to make the banned brand drug list available and further
failure on their part to ensure withdrawal of these drugs from the
market has ensured confusion and non-appiication of the ban (Shiva
1984).
The result of such violation of the ban can be seen in the high
profit made by the multinational pharmaceutical companies that con
trol drug industry in the country. The following table indicates the
amount of profits taken out of the country in comparison with the
total capital investment made.
Table 3: Profit remittances of some multinational companies
Drug Company
Original capital
investment
Glaxo
Rs 1.5 lakhs
Rs 2 lakhs
Rs 3 lakhs
Rs 1.50 lakhs
Rs 4 lakhs
Pfizer
Ciba-Geigy
Cynamid
Bayer
Profits remitted to
parent company in 1980-81
Rs 135 lakhs
Rs 260.82 lakhs (in 3 yrs)
Rs 162.57 lakhs
Rs 157.64 lakhs
Rs 135.82 lakhs
The new drug policy
Unlike the Hathi Committee which systematically reviewed the
pros and cons of various aspects of the drug policy before coming
out with its recommendations, the report of the National Drugs and
Pharmaceutical Council is far from comprehensive. Drug pricing and
fiscal benefits seem to have guided the new drug policy. All other
crucial aspects of a rational Drug Policy have been totally neglected
Our criticism of the National Drug Policy is for the following rea
sons:
The drug policy document as proposed by the steering com
mittee is not based on the actual health needs of the people, the need
for self-reliance in the drug industry, weeding out irrational and
hazardous drugs and a distribution scheme to‘ make drugs availale to
the people at low cost. The policy document consists of only dis
cussions regarding the profitability and viability of the drug industry.
In fact the entire report reads as if it was a forum of bargaining and
investing concessions for various sectoral demands. It is interesting
that the policy document is framed by the government and the drug
industry without representation from the health sector, (governmental
and voluntary) consumer groups, trade unions and other mass
organisations and various scientific bodies. With a composition as
noted above, it is not surprising that the drug policy has been framed
in isolation from a rational health policy.
Surprisingly, a review of the aims and objectives of the drug
policy, 1978 and the DPOO 1979 in respect of the functioning of the
industry for the last 5 years, has not been made. In the absence
of such a review, we fail to understand how a new drug policy can
be formulated. The following glaring ommissions can be mentioned:
No effort in identifying and weeding out of drugs medi
cally accepted as hazardous.
(ii)
No effort at drawing up a National Essential Drug list
to guide the production, distribution and prescription of
drugs.
(i)
(iii)
No effort to make available unbiased drug information to
health personnel and people.
(iv)
No effort to evolve distribution schemes for drugs for
the people.
(v)
No effort to ensure ethical marketing and trade practices.
Even the points discussed by the Drug Steering Committee
do not have the interests of the people at large but have been based
on the needs of the drug companies. For instance, the demand
pattern computed by the Drug Steering Committee and the working
group are not based on the disease patterns existing and the conse
quent need for drugs but on simple extrapolation of existing skewed
demand patterns. No proposal has been made to ensure the produc
tion of essential drugs with appropriate price controls.
With regard to the pricing policy to be followed, the only con
sideration that the DSC has taken into account is the profitability of
the drug industry. The principle that the essential drugs should be
made available at low prices has been paid lip service but no attempt
has been made to formulate policies which will lead to such a result.
The DSC has decided to change the various categories of
drugs and the markups to be provided for each category. However,
no reason has been put forward why the price markups should be
changed The Government of India and BICP (Bureau of Industrial
Costing & Pricing) have yet to produce data that the markups allowed
are leading to losses in the drug industry.
The essential drug list has been reduced to 95 with no re
duction in the irrational and hazardous drugs in the market. Negative
and positive lists have been used elsewhere to guide the selection of
9
drugs in the market. Bangladesh (Rolt 1984) and Mozambique are
very good examples (Shiva 1983). But no such list has been prepared
in India. We have no National Formulary nor Therapeutic Guidelines.
Exclusion of certain basic drugs e g. chlorthiazide as a diuretic
phenobarb and phenytoin as anti-epileptics, pentaimidine for Kalazur,
etc., when 4 general anaestheticsand 4 anti-cancer drugs could be
included, must have required some rationale which we just cannot
find.
The Methodology used for working out demand estimates is
extremely irrational, based more on past warped production patterns
than actual health needs. Consequently, with increased profitability
and liberalisation, further flooding of the markets (rural and urban)
with irrational but more profitable drugs is expected. With most of
the other drugs outside the drug control basket, an increase in drug
prices is envisaged.
With absolutely no change in the number of hazardous drugs
in the market, no effort at ensuring unbiased drug information on
ethical marketing practices and no improvement in quality control
or drug legislation but with ensured profitability, the health of the
drug industry is going to be well taken care of. The tragedy is that
this increased profitabilty is at the cost of the consumers, their scarce
resources and their health. Refusal to swallow a pill, according
to Fritzof Capra, is a political action. Refusal to swallow pills which
are deleterious to healh is the beginning of an important process by
which people begin to reject not only pills but policies such as the one
in the offing.
REFERENCES
Gharpure, Y.H. ‘Drugs for Masses,’ The Eastern Pharmacist, 26 (n. 326, Febru
ary 1983), pp 35-38.
Gopalan, C. Nutrition and Health Care: Problems and Policies. New Delhi:
Nutrition Foundation of India, 1985.
Govt, of India. Report of the Committee on Drugs and Pharmaceutical Indus
tries. yicw Delhi: Ministry of Petroleum and Chemicals, 1975.
10
—. National Health Policy Document. New Delhi: Ministry of Health and
Family Welfare, 1982.
------ . Annual Report. 1984-85. New Delhi: Ministry of Chemicals and Fertili
sers, 1983.
ICSSR and ICMR. Health for All. An Alternative Strategy. Pune: Indian Insti
tute of Education, 1981.
Jayarao, Ramla. ‘Tonics: How much an Economic Waste?
*
Mfc Buileti,.
(November 1978).
Mcdawar, Charles and Barbara Freeze. Drug Diplomacy. London: Social Audit,
1983.
Melrose, Dianna, Bitter Pills. Oxford: OXFAM, 1982.
Narayana, P.L. The Indian Pharmaceutical Industry. Problems and Prospects.
New Delhi: National Council of Appiicd Economic Research, 1985.
Nutrition Foundation of India. The National Goitre Control Programme—A
Blueprint for its Intensification (Scientific Report -No. 1), .New Delhi:
Nutrition Foundation of India, 1983.
Rolt, Francis. Pills. Policies and Politics. London: War on Want, 1984.
Shiva, Mira. People-Oriented Drug Policy: Mozambique Experience. (VHAI
Handout). New Delhi: Voluntary Health Association of India, 1983.
------- . Banned, Banable and Hazardous Drugs. Paper Presented at the All India
Drug Action Network Meet, Wardha. New Delhi: Voluntary Health
Association of India, 1984.
UNICEF. An Analysis of the State of the Childrenin India. Geneva: UNICEF,
1984.
WHO. The Selection of Essential Drugs. Geneva: Technical Report Series No.
615, 1977.
Repeated Frpm
SOCIAL ACTION Vol. 35 No. 3 July-September 1985
11
40, INSTITUTIONAL AREA, SOUTH OF l.l.T.
NEW DELHI - 110016
D.
1 668071,668072
Phones j 66501g, 655871
VOLUNTARY HEALTH ASSOCIATION INDIA
ALL INDIA DRUG ACTION NETWORK
RATIONAL DRUG POLICY
CAMPAIGN
January 22, 19E6
Dear Friend,
Several organizations deeply concerned about the drugs issue have been
making a concerted effort for several years towards ensuring the formulation
of a Rational Drug Policy.
In view of the new Drug Policy coming up in the Parliament in the coming
session, it is imperative that socially conscious individuals, health pro
fessionals and organizations make a clear statement regarding the kind of
National Drug Policy that we want for our nation.
We want a rational people-oriented Drug Policy, which ensures :
1.
Easy availablity of essential and life saving drugs at all levels.
This is the basic aim of the Rational Drug Policy.
Today shortages of anti-TB, anti-leprosy, several other drugs and
even iodized salt exist. Production of Vitamin ‘A’ is steadily declining
while over 40,000 children are becoming blind each year for lack of Vitamin
'A'. Adequate production and streamlined distribution of essential drugs
must be ensured.
2.
Withdrawal of hazardous and irrational drugs.
Over 43,000 formulations are sold in India. This large number of for
mulations not only creates confusion in the minds of consumers, doctors,
and chemists but also confuses the licensing and drug control authorities.
Several thousands of these formulations are known to be irrational,
hazardous and are banned in other countries. Drugs not allowed to be
produced in the parent countries of the manufacturing multinational
companies are being made and sold here.
It is critical that we throw out irrational and hazardous drugs; that we
do not clutter and clog the drug control mechanism.
3. Need for Drug Legislative Reforms—so that legalistic loopholes
are not used against the people repeatedly and systematically.
The EP case is the cruellest of such examples. In 1976 a warning
against use of High Dose Estrogen-Progersterone combinations for pregnancy
testing had been issued, as these drugs were found to be associated with
foetal malformation.
In 1982, the Drug Controller of India banned high dose EP combinations
vide DO No. X19013/8/81-D.
Organon, Nicholas and Unichem went to court and managed to get a
stay order against the ban-A stay order which has to date not been
challenged. The sales of these banned drugs still countinue.
A point to note is that Organon (new calling itself Infar) is not allowed
to market high dose Estrogen-Progesterone combination in its home country
Netherlands.
4. Availability of unbiased drug information to health personnel
and consumers. This is basic to Rational Drug use.
Today health personnel depend heavily on drug companies and their
representatives for drug information. The drug information given to
doctors for the same product of the same company differs in developing
countries like India and the developed countries—with clearly shows
existence of double standards.
5.
Effective Drug Quality Control.
Every fifth drug in the Indian market is substandard. Allowing pro
duction and sales of substandard, essential and life-saving drugs is a crime
against the consumers. Quality control mechanisms need to be overhauled.
There is a need for more quality control laboratories.
We need more qualified, and trained and competent drug inspectors for
for drug quality control. Co-option of consumer bodies is also needed to
ensure drug control. Use can also be made of quality testing facilities of
teaching institutions,
You are requested to join the Rational Drug Policy Campaign and
do the following :
Health Ministry
1.
Write to : Mrs. Mohsina Kidwai, Minister for Health and Family
Welfare, Nirman Bhawan, New Delhi-110001.
(
3
)
asking for the stand of the Health Ministry regarding the drug
policy.
ii) asking the Health Ministry to take a leadership role in for
mulating a Rational Drug Policy and not absolve itself of
its responsibility.
i)
2.
Dr. Harcharan Singh
Advisor, Health and Family Welfare Planning Commission, Yojana
Bhawan, Parliament Street, New Delhi-110001.
........ for the same.
3.
Director General Health Services
Nirman Bhawan, New Delhi-110001.
for the same.
Ministry of Chemicals and industry
4.
Mr. R. K. Jaichandra Singh
Minister of State for Chemicals and Petrochemicals,
Shastri Bhawan, New Delhi-110001.
—asking him to clarify to the nation why the drug policy is looking
only at the pricing and production aspects totally ignoring other
aspects needed to rationalize the nation's drug policy.
5.
Mr. N. D. Tiwari
Minister for Industries, Udyog Bhawan, New Delhi-110001.
B.
Boycott all Banned and Bannable products and all the products
of companies like Organon (Infar), Nicholas and Unichem which
are flouting our laws.
C. Approach the Parliamentarians in your state about safeguarding
people’s interest when the policy comes in the House.
(For list of Parliamentary Drug Consultative Committee and a note
on how to use the Parliamentary procedures, please contact me).
D.
Find out more about the drug and health issue and share the
information with others, help in distribution of the drug campaign
material produced by All India Drug Action Network comprising of:
Arogya Dakshata Mandal, Pune.
Catholic Hospital Association of India, Delhi.
Consumer Education & Research Centre, Ahmedabad.
(4) Consumer Guidance Society of India, Bombay.
( 5 ) Drug Action Forum West Bengal, Calcutta.
(6) Delhi Science Forum, Delhi.
( 7) Kerala Sashtra Sahitya Parishad, Kerala.
( 8 ) Locost, Baroda.
(9)
Lok Vigyan Sangathana, Bombay.
(10)
Medico Friends Circle, Pune.
(11)
Voluntary Health Association of India, Delhi.
(1 )
(2)
(3)
E.
Ensure effective drug distribution of essential drug and vaccines,
iodized salt to the peripheral areas.
While vaccines are unavailable or available with the cold chain not
being maintained. It is criminal to allow children to be crippled and
maimed for life because as a nation we are unable to ensure an
effective drug distribution system even 38 years after we have had
the total freedom to revamp our health and drug policies.
The new Drug Policy is coming in March. It is mainly looking at drug
pricing and production and will safeguard the interest of the greatest in
fluencers of our drug policy i.e. the drug industry.
We want the following to be considered in the formulation of the
National Drug Policy :
— WHO's recommendation and criteria for a Rational Drug Policy.
—Conclusions of two Summits of Heads of State of Non-aligned
nations — Havana and Colombo.
—The recommendations of ICMR—ICSSR report 'Health for all by
2000 AD.
AIDAN's and VHAI’s stand is along the lines of the above national and
internationally accepted conclusions.
Drugs cannot become a substitute for basic health needs for food, water
and safe hygenic living and working conditions.
Hazardous, irrational and substandard drugs cannot become substitutes
for life saving essential and quality controlled drugs.
DRUGS POLICIES SHOULD HAVE MORE TO DO WITH
HEALTH AND LIVES OR PEOPLE, THAN PROFITS FOR
THE DRUG INDUSTRY.
People have the right to reject irrational and hazardous drugs and
policies.
PLEASE EXERT YOUR RIGHT I ACT FAST NOW, WE URGENTLY
NEED YOUR SUPPORT !
— Dr. Mira Shiva
Coordinator
All India Drug Action Network
and
Low Cost Drugs & Rational Therapeutics
%
A to
advertising
OF DRUG POLICY ISSUES
Oho of the main tools of drug companies to create
a need for their products. Includes appeals for status,
modernity, even unnecessary uses and cosmetic embellishments.
BULK PURCHASING
Buying of drugs in bulk by competitive buying of generic
drugs in the international market. It does away withbrand
names and private importers.
BULK DRUG
This is the basic, active chemical ingredient of a drug
formulation.
BASIC RESEARCH
This implies fundamental and innovational process or
product research. A necessary step to prevent dependence
on foreign companies.
DIO—AVAILABILITY? This means that the same chemical ingredient, may be
therapeutically different because of tho way of formulation.
A common but unconvincing argument against generic
proscribing by drug companies.
An Important and growing need in the formulation of a
CONSUMER ALERT
ANO CONSUMER
ACTION
safe drug policy.
DUMPING
Passing of old, unwanted, out-dated and banned or
otherwise inferior products on an unsuspecting public.
A common practice of multinationals operating in third
world countries.
COMMUNITY HEALTH CELL
47/1,(First FloorJSt. Marks Road
BANGALORE -560 001
,,
.2
|2»
DRUG PRICE CONTROL
ORDERS
Government orders to control prices of drugs and
profits Issued in 1963, 1966 and 1970 and 1979,
ESSENTIAL DRUG
LIST
A list of medicinal products of proven efficiency,
acceptable safety and suitability to satisfy the
health needs of the majority of the population.
FORMULATIONS
These are finished products thich are directly consumed
and contains in addition to the active drug compound
other ingredients such as diluents, binders, flavouring/
colouring agents, gelatin shells, chemical bases and
waxes and preservatives.
FOREIGN COMPANY
In India defined as a company with foreign shareholding
exceeding 40% of the total share capital.
GENERIC PRESCRIBING
Prescription of drugs using the official, international,
non-proprietary name and not trade or brand namos. eg,»
Aspirin not Aspro or Plusprin,
HATH I;.. COMMITTEE
REPORT
An exhaustive report of far reaching importance, by the
Committee on Drugs and Pharmaceutical Industry, Government
of India, published in April 1975,
.3
83?
HERBAL F.EOrCIHES
fl rich source of medicines for all the common ailments
*
Forms part of the rich cultural heritage of many countries.
Vietnam and China promoted them as part of their drug policy.
INTERNATIONAL CODES These are codes of quality or safety of products or
business procedures, eg., Code of Ethical Marketing Practices
of Health Action International, fln important step in
pressurising multinationals to stop exploiting the third
world.
*
«JUhK
DRUGS
These are newer drugs in the market whose only additional
value are cosmetic embellishments, elegant packing,
irrational combinations all of which help to increase its
cost.
KNOW-HOW
An important requirement for the technical improvement
of tho drug industry. Often controlled by patent, royalty
rules and monoply of foreign conpanits.
LOFOTIL
fl good example of drug sold in India which is banned in
most countries. Especially dangerous for use with infants.
WO manual dismisses the drug as of no value.
RE—TOO DRUGS
These are products of r<search using molecular manipulation
which are profitable but not necessarily a scientific
advance.
.............
Is tho hike in the price above the basic production cost
MARK-UP
and is the amount used for selling costs, administrative
expenses and profits. It is presently fixed by government
orders. The less essential the drug formulation
higher tho mark-up is allowed in India,
MET UORTH
RETURNS
Is an expression of the profit potential of a drug company
and is one of the highest in the chemical industry in India.
It makes drug companies one of the most attractive investments
in the organised industrial sector of the Indian economy.
ORT or ORAL
REHYDRATION
THERAPY
A very important need in tho rational management of diarrhoeas
in children and its popularity will prevent use of many
anti-diarrhoeals that have doubtful therapeutic value.
PACKAGING
The art of packing drugs not only to ensure integrity of
product but to give it ’brand distinctiveness’ and
V
’consumer appeal
.
*
PUBLIC SECTOR
Helps to increase cost of the drugs.
Includes drug manufacturing companies owned by the central
government. These have pioneered the production of bulk
drugs in the country. The government policy attempts to give
it a leadership role.
QUALITY
CONTROL
A very important step in manufacture and distribution of
drugs, m India this ie organised by the Drug Controller
*
:5:
RATIONAL DRUG
THERAPY
A mat hod of prescribing which io efficient, safe, low cost
and easy be administer.
RESEARCH AND
DEVELOPMENT
Tr&d)
A much neglected area in the drug industry. A very necessary
requirement for a country to evolve its otn indigenous
drug policy.
SALES PROMOTION
Techniques aimed at consumers, dealers or intermediaries
to increase short term sales and inspire good will. For
drug companies it includes bonuses with purchase, contests
and competitions, samples and give ways.
SAMPLES
Commonest method by drug companies to woo doctors. Other
methods are advertising in medical journals, leaflsting,
sponsoring medical events, hospitality and providing gifts.
TRANSFER PRICING
Importing of raw materials from parent companies by
multinational subsidiaries at rates higher than the prices
in the international market thereby transfaring cost to tho
local consumer.
U.N. AGENCIES
These include UNIDO, UNCTAD, UNICEF. All Of those are
gearing up to help developing countries evolve relevant drug
policies.
>6
VOLUNTARY ACTIOW
ONLY VOLUNTARY action and initiative can tackle
many drug policy issues. The GONOSHASTHYA KENURA
and G.K. PHARMACEUTICALS are one example op such an
initiative.
L'ORLQ HEALTH
ORGANIZATION
Their expert committee reports and working group
reports are providing the technical back-up for the
evolution of a more health oriented policy in
member-nations.
^’4
■
medico friend
I 11 4 circle
■
bulletin
JULY
1984
RATIONAL D)RO@ POLICY
A Drug Action Network Memorandum
WE, the health personnel and citizens of India
recognize health as a fundamental
right of the
people in this, our welfare state. We recognize and
strongly believe that the health status of our people
is more dependent on their access to adequate food,
safe and adequate water, proper sanitation and clean
environment.
WHILE we support the overall perspective and
approach of the new National Health Policy State
ment and demand its proper implementation, we
believe that, a Rational Drug Policy is an integral
part of a good National Health Policy.
WE, therefore, demand the following:
3.
We have a right to safe, essential, quality
drugs which are in keeping with the health
needs of the people, at costs which the
majority can afford.
We urge our government to accept and
implement the Hathi Committee Recom
mendations which are also in keeping with
the WHO Guidelines for a Rational Drug
Policy.
Further the national drug formulary should
be revised and compiled by an expert multi
disciplinary committee keeping the follow
ing criteria in mind:
Essentiality
Efficacy
Safety
Cost
Ease of administration
Availability
Potential for misuse
Such evaluation of the drugs in the market
and revision of the lists should be done
periodically.
The Essential Drugs Policy should be adop
ted for all health services, government and
private, and priority in production, distri
bution and dispensing should be given to
these essential drugs.
GriVThe public sector should^i^djtg^'elsen^lRosA
"sOO
6.
7.
S.
9.
10.
and life saving drugs on a priority basis at
the national level.
Drug production by multinationals and
private manufacturers in India should also
be aligned with national health priorities.
Bulk procurement of essential and needed
drugs should be through world-wide com
petitive tenders and rationalization of drug
purchases should govern both the public
sector as well as private health sector.
Imports and production of non essential,
specially hazardous drugs, should be strictly
curtailed.
Drugs which have been banned from sale
after being marketed for some time in one
country may not be submitted for clinical
trial or marketing in India. The onus of
proving why a non-essential drug should be
introduced or allowed to continue on the
market should be with the manufacturer
and such introduction should be preceeded
by adequate trials and evaluation by Drug
Control Authorities.
Comprehensive drug legislation which covers
areas such as price control at different
levels, patents, and
marketing practices
should be incorporated to serve the objec
tives of the national drug policy and there
INSIDE
Medical care — a critique and beyond
3
Dear Friend
6
Keeping Track
6
Drug Alert
7
Vocal Figures
7
Editorial
8
QY, renew yoar ^4iscrtj>hG.i
should be levies, sales tax or excise duty
on any pharmaceutical
product in the
essential drugs list by the Central or State
governments.
11.
No technology transfer agreement shall be
legal and binding which contains restrictive
practices, disproportionate and unneces
sary use of imported intermediaries or
obsolete technologies or unfair arrange
ments with respect to prices, payments or
repatriation of profits.
12.
The National
Drug Policy, should state
clearly the steps towards a complete aboli
tion of brand names and as a first step use
of generic names should be made compul
sory in medical education, prescribing and
labelling of drugs. Generic names should
appear more prominently on all packagings.
13.
It shall be the primary responsibility of the
manufacturer to ensure the quality of drug
products. However, it shall be the statu
tory responsibility of the Drug Control
Authorities to monitor the standards and
ensure a minimum uniform level of govern
ment control. Consequently, the govern
ment shall take all necessary measures to
enable the Drug Control Authorities to
function in an effective manner and dis
charge the statutory duties thrust upon
them.
14.
15.
It shall be the statutory duty of the drug
control authorities to inform health person
nel and consumers, of the essential drugs
lists, policies, categories or brands of
drugs banned for
manufacture or sale,
through publication in the national news
papers, magazines, and medical journals
with adequate explanations and details.
Availability of drugs required in the Gov
ernment’s National Programmes should be
ensured on a priority basis to the govern
ment as well as voluntary and private health
institutions. Quotas for anti tuberculosis,
anti leprosy, anti malarial drugs, iodized
salt etc., should be made easily available
with regularity of supply to the voluntary
health institutions where ever possible,
ANNOUNCEMENTS
I.
Leadership Development Course in Commu
nity Health and Development 9 September
to 23 October 1984.
A Course for non-doctors in existing commu
nity based programmes—designed to provide
knowledge and skills to promote building of
communities. For details write to Deena-
specially when their performance in health
care delivery is known to be effective.
16.
In all review committees, statutory bodies
and other such bodies, there should be ade
quate representation of consumer groups
and the voluntary health sector.
17.
Drug companies should follow ethical
marketing practices,
and this should be
ensured by their own organizations like
Organization of Pharmaceutical Producers
of India (OPPI), Indian Drug Manufac
turers Association (IDMA), International
Federation of Pharmaceutical Manufac
turers Association (IFPMA). We deplore
the tendency of these companies and asso
ciations to get round every progressive mea
sure of the government through recourse
to technicalities of the law and through
the courts.
18.
The marketing code drawn up by Health
Action International (HAI) should form
the basis for a National Code for Marketing
Practices. This should be accepted by our
government and should be suitably imple
mented through legislation.
19.
The government of India should take a lead
and endeavour to influence the WHO and
the WHA to adopt the Code in the interests
of the other developing countries and their
people —
— Voluntary Health Assocation of India
— Centre for Science and Environment
— Centre of Social Medicine and Com
munity Health, Jawaharlal Nehru
University
— Kerala Sastra Sahitya Parishad
— medico friend circle
— Arogya Dakshata Mandal
— Lok Vigyan Sanghatana
— Consumer Guidance Health Services
— Consumer Education Research Centre
— Federation of Medical Representatives
Association of India.
bandhu Training Centre, R. K. Pet, 631 303
(Tamil Nadu).
Continuing Education for General Practitioners
A self-paced correspondence course for MBBS
doctors/General Practitioners to up/date
knowledge and skills for better patient care.
For details, write to the Coordinator, Conti
nuing Medical Education, Christian Medical
College, Vellore 632 002 (Tamil Nadu).
MEDICAL CARE — A CRITIQUE AND BEYOND
Satchidanandan, Calicut
I
We shall begin with an examination of the basic
tenets of the political-economic critique of modern
medical and health care projects. The politicaleconomic critics do not dispute the benefits of modern
medicine; only they want the weaker sections of the
society to have easier access to its unalloyed blessings.
Poor or unequal geographic distribution of doctors
and hospitals, lack of proper service in the rural areas,
low technical quality of service, discriminating treat
ment based on race, caste or sex, insufficient alloca
tion of funds for medical care in government budgets,
non-availability of necessary medicine, the general
urban orientation of medical services, the exploitation
of underdeveloped countries, especially of the third
world by transnational corporations, chemical and
technical experiments performed on colonial people
by imperial masters, the unethical use of medicine in
war and in the promotion of imperial interests —
these are the recurring issues raised by these critics.
The solutions to these problems are also politicaleconomic: creation of an organised health care system,
government sponsored mechanisms to promote a
more equitable distribution of and better quality in
health care, greater centralization and careful bureau
cratization . The more radical of these critics also find
fault with all kinds of private ownership and control
of medical and paramedical
institutions. They
demand complete control or even the pros
cription of the profit
oriented private clinics
and medical supply companies — in short a sociali
sation of.medicine on the models of Soviet Union or
the countries of Eastern Europe.
A WORKER SPEAKS TO A DOCTOR
We know what is it that makes us sick.
They say you are to treat us when we are ill.
They say you have learnt medicine for ten years in
excellent institutions built on the sweat of the
people
And you have spent large sums to learn your art.
Then you should be able to cure us, but can you?
When ive Visit you, you take off our rags and probe
our naked little bodies
What is it that you are looking for?
■— the cause of our illness"!
you would know better by looking at our rags.
It is the same disease that consumes
our bodies and our clothes.
You say our shoulders ache because of moisture,
the same has stained the walls of our huts.
Now tell us, where does this moisture come from?
A little food and a lot of work
make us pale and weak.
Your prescription says: put on more weight.
It would be better to tell a blade of grass
not to get wet in the rain.
How much time will you give us?
We see that a single carpet in your mansion
costs the fees you get from five thousand patients.
You will plead innocence, sure.
The stain on the walls of our hut
has the same tale to tell, too.
— Bertolt Brecht
The political-economic critique, we should admit,
is still not entirely irrelevant in the semi-colonial and
underdeveloped situation obtaining in India. The
enviable prestige that doctors enjoy in a generally
illiterate country, their high status and money making
capacity are enough temptation for any parent to
wish to secure an admission for his child in a medical
college even at the cost of honesty. The primitive power
of a patriarchal society continue to drive even the dis
inclined to the profession resulting in technical incom
petence. The unwholesome alliance between pharma
ceutical distributors and physicians and the callous
export of useless or even harmful drugs by their
global producers and corruption rampant at all levels
of health services make the situation still worse.
There is also a disproportionate emphasis placed on
hospital oriented curative medicine compared to pre
ventive medicine and environmental hygiene.
The worker in this poem is pointing to a serious
disease that has come to afflict the physicians in our
country too: their absolute alienation from the com
mon people whose toil have made them what they
are. Careerism, callousness, corruption and greed are
fast turning many of our doctors into a class of dubi
ous integrity. But 1 refuse to consider the situation
absolutely irredeemable. I do not mean to suggest
that every doctor should transform himself into a
Che Guevara or a Norman Bethune, but there is no
reason why young idealists, that many of you cer
tainly are, cannot switch on a programme of house
cleaning, a revolution of the scalpel and the
stethoscope.
What will be the nature of this medical revolu
tion that I wish you would set your minds to? What
are its theoretical premises and its understanding of
itself? What realistic goals and practical programmes
can such a movement for socialist medicine have? I
am presenting before you a very sketchy outline for a
total critique of modem medical practice from a
socialist perspective and the programmatic promotion
of a counter-medical culture.
It is wise to remember that the majority of the
diseases that attack and kill our people are of an
infectious nature like tuberculosis, diarrhoea, dysen
tery, typhoid, leprosy and cholera or are' mental dis
orders resulting in psychosomatic dysfunctions that
spring from the tensions natural to our social mili»u.
But we have modelled our medical development on
3
means of social control. The unlimited growth of
medical care also threatens to destroy the environ
mental and cultural conditions needed by people to
live a life of autonomous healing. Medical technology
has been helping industrial growth rather than per
sonal growth. He admits that chemotheiapy has
played a significant role in the control of pneumo
nia, yaws, malaria, tetanus, diphtheria, scarlet fever
and sexually transmitted disease but it'has contri
buted little, he argues to the decline of mortality or
morbidity from these diseases. Even further the pain,
dysfunction, disability and anguish resulting from
technical medical intervention have contributed to
the increasing morbidity of modern life. The un
wanted side-effects of medicines have alsd increased
with their power. Many drugs are addictive, mutila
ting or mutagenic; antibiotics can at times alter the
normal bacterial flora and induce a supcrinfection;
other drugs help breed drug-resistant strains of bac
teria. The overuse of dangerous diagnostic proce
dures, the administration of synthetic hormones,
chemical stimulation of labour, ultrasonic fetal moni
toring, the routine use of anaesthesia for delivery,
overdose of powerful drugs like chloramphenicol, using
drugs in dangerous combinations, medical treatment
of non-existent diseases and unnecessary surgery have
a disabling effect on their victims. Professional call
ousness and negligence arc increasingly being attri
buted to a break down or absence of equipment;
thus moral faults come to be justified as technical
errors. This aspect of the problem lllich calls ‘clini
cal
iatrogenesis’. Medical practice also sponsors
sickness by encouraging people to consume curative,
preventive, industrial or even environmental medi
cine. Defectives are prevented from work and prom
ptly removed from the scene of political struggle to
reshape the society that has made them sick. This
invalidating process is what lllich calls ‘social iatro
genesis’. Health professions also destroy people’s
capacity to deal with their human weakness in a
personal and autonomous way. Birth and death are
equally controlled and culturally mediated by medi
cine. Death is turned into a profitable commodity
with endless potential. Health management is desig
ned on the engineering model. This has been termed
‘cultural iatrogenesis’.
1 would like to add two more modes of iatro
genesis; one is philosophical. Modern western medi
cine suffers from an overdose of scientism, an extreme
form of mechanical materialism. First, it follows the
doctrine of specific etiology, where the existence of
a cause is mechanically connected to the disease.
Second it conceives the human body as a machine,
the functioning of whose parts is considered inde
pendent of the mind of the organism. It does not
take into account the interactions of body, mind, and
physical and social environment. Thus’ there’is a
disassociation of mind and body in this medical technologism. Both these can be traced to the capita
listic formal logic that governs medical science. Only
a dialectical approach can make medicine a genuine
science. The medical technology itself is capitalistic;
western lines concentrating on non infectious diseases
like diabetes, cancer, hypertension and cardiovascular
diseases, more prevalent in the developed countries.
Most of the communicable diseases are bred and
nourished in India by conditions of underdevelop
ment and can be considerably controlled by improve
ments in the living standards of the poor.. Better
food, facilities for better housing and clothing, regular
supply of disinfected water, more leisure and facili
ties for recreational activities, cleaner surroundings
and a peaceful environment alone will put an end
to the contagion that make our hospitals overcrowded
hell.;. The struggle for the prevention of diseases has
thus become an increasingly political question related
to the removal of exploitation and the establishment
of a genuinely socialist society. Modem statistical
studies by Rene Dubos. Thomas McKeown, John
Prowles and A. L. Cochrane have proved beyond any
doubt that the fall in the death rates and the decline
in contagious diseases recently observed in the deve
loped countries have little to do with curative medi
cine. They are products first of better nutrition and
housing facilities, second of improvements in control
of the environment and only third of personal medi
cal attention. 1 know that this has been formally
recognised by our national health programmes and
that social and preventive medicine has been formally
included in our medical syllabi. But the question is
how far does this awareness inform the real activities
of our doctors and health forums. What is their
cumulative contribution to the study and dissemina
tion of epidemiology, medical sociology and environ
mental education? I think this is precisely a field
where informal and non-bureaucratic medical move
ments can make great strides.
II
The socio-cultural critique of medical practice
is a more recent development. Ivan lllich, the Vien
nese thinker who has developed a series of institu
tional alternatives for technological societies was
perhaps the first to develop a consistent and radical
cultural evaluation of western medical care. Besides
lllich, feminists like Linda Gordon and Pauline Bart,
black radicals like Frantz Panon and medical socio
logists like Irving Zola and John Ehrenreich have
helped to create a profounder understanding of this
cultural crisis. lllich opens his book, ‘Limits to Medi
cine’ with the words “The medical establishment has
become a major threat to health. The disabling
impact of professional control over medicine has
reached the proportions of an epidemic”. He calls
this epidemic, ‘Iatrogenesis’ meaning a disease born
of physicians. lllich holds that it is the layman and
not the physician who has the potential perspective
and effective power to stop this-epidemic (though 1
see no reason why earnest and intelligent physicians
cannot join hands with the layman in an attempt to
demystify medicine). The increasing ‘medicalization of
life’, lllich argues, is a form of the colonisation of
the body. By holding the exclusive right to deter
mine what constitutes health and sickness and what
shall be done to them, medicine has become a major
4
that is why .a mere socialisation of medicine cannot
make it socialist. The other mode is moral iatroge
nesis . Professionalism in medicine has unfortunately
come to mean a defence of occupational and class
privilege rather than high standards. The profession
thus has created an elite fatally cut off from the
public, speaking a highly specialised language meant
to mystify the layman. The cruellest examples of
moral iatrogenesis may be found in psychiatry which
openly practices the social control of deviant beha
viour. It is concerned not with clinically measurable
somatic dysfunctions but with what is socially defined
as abnormal or unacceptable behaviour. R.D. Laing’s
classic example may prove my point. A man gibber
ing away on his knees, talking to some one who is
not there should normally be considered mad; but
society has come to define this activity as ‘prayer’ sb
that we consider it perfectly sane. Psychiatrists have
the power to label several states ranging from rare
creativity to revolutionary activity as forms of
insanity. They help the preservation of the statusquo by silencing its opponents as in the Soviet Union.
Our hope here lies in the development of anti
psychiatric movements trying to discover the social
roots of abnormal states and discouarging monstrous
methods of treatment such as ECT, replacing them
with love, understanding and patient persuasion.
campaign should enable the people to ask questions
to the doctors on terms of equality and judge the kind
of care and treatment offered to them. Secondly
the new movement can see to the enforcement of the
oft-broken medical code of conduct. This should be
realised as far as possible, by persuasion, moral
authority and honest example rather than direct
coercion. Thirdly, the forum can conduct or guide
revealing studies in the geography, history, sociology
and politics of medical care in India, particularly in
the States. These studies can expose the various
forums of institutionalized corruption and expose the
sexist, casteist and class prejudices now inhibiting
medical study and practice and the effects of colonia
lism on our attitudes to illness and cure, thus evolving
a total critique of medical practice in the country.
Fourthly it can propose alternatives to the present
medical syllabi so that they may place greater empha
sis on a realistic study of our environment and study
of the doctor-patient relationship. Healing relation
ships are as much social as they are physiological; so
chemistry, biology and physics alone cannot form an
adequate basis for scientific medicine. It should find
place for subjectivity and consciousness in the study
of man so that it becomes a dialectical science. Tradi
tional medical systems like tribal cures, indigenous
and holistic systems like Ayurveda and non-allopathic
systems like nature cure, homeopathy, Unani medi
cine and acupuncture should form a part of the
syllabi. The forum can also encourage researches in
mixed medicine that integrates the various approa
ches. Fifthly it can assist the deprofessionalisation of
medicine, by teaching the patients to conduct their
own laboratory tests vand offering compressed courses
in environmental hygiene and preventive medicione to
volunteering youth as is done in the case of the ‘bare
foot’ doctors in China. (Books like ’Where there is
no doctor by David Werner can be of use in such
courses). It should simultaneously ‘reprofessionalise ’
medical care by invoking the idea of health care as
a calling and a selfless mission. Sixthly and lastly,
it can also build up a parallel system of clinics and
nursing homes where the foundation is laid for a new
type of doctor-patient relationship and a novel
approach to the problems
of medicine including
psychiatry.
HI
The political-economic critique and the socio
cultural critique of modem medicine are not as con
tradictory as they may appear to be. If we can un
leash the imagination of the people and lay the foun
dations of a popular movement it must be possible
to bring about a synthesis of the perspectives of
required in the
‘more’ and ‘different’. What is
Indian situation of scarcity and corruption is an
equal emphasis on the need for more services
and the need for a different approach to health
altogether. The seed of such an ambivalent popular
movement for socialist medical care can be sown now,
in the form of a forum of radical medical
students, teachers, practising doctors, psychiatrists,
non-professional health workers and active sympa
thisers. The primary task of this forum will be cons
tituted by campaigns of demystification and conscien
tization. The aim of these campaigns will be the
demedicalization of society by spreading the concepts
of self-help. But we should take care to see that it
does not promote superficial fads. Some medical
’but
inappropriate
for
technology
is
useful
Rejecting this would
use by utrained people.
of
‘
autonomy
’
.
What
be a self-destructive form c. ___
is required is more a reorientation of dependency,
rather than a complete abandonment of dependency.
Conscientization can be done through the publication
of books, pamphlets and periodicals and by camps
and classes meant for weaker sections based on the
dialogical and problem-posing
modes of socialist
pedagogy as outlined by Paulo Freire. The whole
Courtesy: Calicut Medical College Magazine 1982-83
Health for all-depends on three things: Reduc
ing poverty and inequality and spreading education;
organising the poor and underprivileged tofight for their
basic rights; replacing counterproductive consumerist
western model of health care by alternative model based
in the community.
— ICMR/ICSSR
5
KEEPING TRACK
DEAR FRIEND...
Tuberculosis — Annual Meet theme: 1985
1.
Rakku’s story: Structures of Ill Health and
the source of Change. Sheila Zurbrigg.
An analysis of the Indian health care system
and of the basic source of change. 250 pages.
Rs. 10-00 (US $5).
What is the purpose of this exercise?
— Is the purpose to do a critical analysis
alone and leave it at that?
Or .
— Discuss the various dimensions of the
TB problem for our own understanding
and for education of others in mfc.
Or
— Discuss the TB issue from the point
of view of the workers in the field of
health with the purpose of ensuring
some improvement ie., action plans
being an important aspect of our work
-— this would obviously be based on
the above two but would mean our
going beyond that.
In C1NI this issue had come up, as to the chang
ing role of mfc and I strongly feel, unless we are to
some extent involved in coordinated action over selec
ted priority issues, we will stay this big and have very
marginal impact.
I feel extremely strongly about the TB issue and
I’d hate to see the whole thing limited to an intellec
tual exercise, no matterTiow fantastic.
Mira Shiva
VHAI, New Delhi
(1)
Available from Centre for Socal Action, Gundappa Block 64, Pemme Gowda Road, Ban
galore-560006.
2.
Rational Drug Therapy — a small book on
recent advances in the treatment of common
diseases, rational approaches to treatment,
side effects of various drugs and clinical diag
nosis of common diseases by various experts
in the field.
To be published by Arogya Dakshata Mandal.
Register orders with Dr Patwardhan of Pune
Journal of Continuing Health Education.
1913, Sadashiv Peth, Pune 411030.
3.
Socialist Health Review (quarterly) A new
collective' effort which aims to provide a forum
for exchange of ideas and for generating a
debate on practical and theoretical issues in
health from a radical or marxist perspective.
For further information and subscription
(Rs. 20-00 for individuals and Rs. 30-00 for
institutions) write to Socialist Health Review,
19 June Blossom Society, 60 A, Pali Road,
Bandra (West) Bombay 400 050.
Actual discussions should focus on the follow
ing issues: •
a)
Where does TB fall in our priorities?
b)
What priority in exact terms has been
accorded to it in our national, state
level, hospital level and day to day
practice as well as in medical education?
c)
assessment, remarks, criticism in the
current direction of approach to the
problem of TB.
d)
What could be the rational approach
at each of the above mentioned levels?
We should avoid discussing details of chemo
therapy etc., if we are to encourage participation of
non-medical people in mfc.
Current concepts and WHO recommendations
about diagnosis and chemotherapj' and the evolution
of the care of TB at our national level may be cir
culated through background papers and the bulletin.
Kartik Nanavati
Ahmedabad
(2)
4.
Diarrhoea Dialogue — a quarterly newsletter
published by AHRTAG (Appropriate Health
Resources and Technologies Action Group
Ltd), London. It shares practical information,
experiences and field studies on child health
with special reference to diarrhoea.
(Wc have received a letter from Dr William
Cutting, one of the editors, offering to pul
readers of mfc bulletin on the free mailing
list. Those who are interested, please send us
your names with postal address (in capitals)
by 31st July 1984).
Self-sufficency in health care
A community health project in-charge, pre
ferably a lady with minimum 2 to 3 years
experience in community health nursing and
rural development activities is required imme
diately by ANKURAN, a self-reliant health
project
in Gudri Mahalla, Chatra, Dist.
Hazaribagh, Bihar 825 401. Send applications
with complete bio-data, copies of certificates
and a passport size photograph to Sri Anand
Kumar, its Secretary.
(3)
The main article in June’s issue “Towards an
Appropriate Strategy’ was very interesting for us as
this is the sort of work we are starting to try to do.
I wish it had been more detailed, in fact.
Please keep up the immensely good work that
mfc does. It is one bright patch in an otherwise very
gloomy picture.
Keith & Caroline Walker
Gangavarpatti, T.N.
6
?f-1
COMMUNITY HEALTH CELL
47/1,(First FloorlSt. Marks
BANGALORE - 580
3-4/373
k/17/ 7/ 84
CRITERIA OF A RATIONAL DRUG POLICY
(Background paper prepared by Dr, Sujit K. Dass &
Others, Calcutta for DRUG ACTION NETWORK (DAN)
Core Group meat, Wardha - July 30-31, 1984.)
Drugs may bfe defined as substances used in the exercise to
alleviate physical and mental ailments of living body as well as
to prevent illness.
Hence, the use of drugs is a part of the
health care service for the individual .and the community.
India
has no precise or comprehensive policy on health care service.
A drug policy cannot but be an integral part of the health care
policy.
The basic elements of desirable health care policy for
the country should, therefore, be, identified before we discuss
the criteria of a rational drug policy.
Majority of the Indians suffer from the diseases of poverty and
ignorance i.e. Communicable diseases, diseases due to undernutri
tion, etc. These are preventable and curable.
Industrialisation
and urbanisation have also led to spread of consequential diseases.
What we need then, is adequate nutrition, safe water, universal
sanitation, environmental protection and a primary medical care
service available to all.
Obviously, this is a tall order at
present. Keeping therefore, all socio-economic-political-cultural
constraints in mind, the following should constitute the prio
rities of our health policy:
1.
Universal immunisation programme.
2.
Free clinical service to all indigent people (.people living
below a pre-determined level of income).
3.
Provision of adequate Calorie for the indigent people.
4.
Planned extension of sanitation and water supply.
5.
A rational drug policy.
These priorities may appear to be inadequate and imperfect.
But
these have been set up with a view to confine within achievable
goals.
Role of drugs
To the people, doctors and non-doctors alike, drugs appear as
panacea for all ills.
Health is still regarded as an individual
or personal responsibility and it is believed that freedom from
diseases could only be obtained by better and better and more and
more drugs.
Such a belief, among educated and illiterate alike,
h’S led to a universal•craze for drugs and the DRUG CULTURE has
come to dominate the society.
On the other hand another school,
comprised of obscurantist and progressives, point out the harmful
consequences of the drug culture and prescribe a journey back to
nature.
We are cold to reject this dependence on drugs and con
centrate on attacking the soci-economic causes of illness.
It is not disputed that without being free from inequality and
exploitation, a society cannot achieve freedom from avoidable ill
ness.
Cn the other hand, one cannot allow people to suffer and
die till that real freedom arrives.
Hence, in
*
our society, medical
care service has an immediate, essential and pfioritv role to play
and the drugs occupy a pivotal position in the medical care service.
Besides, there are preventive drugs too e.g., vaccines etc.
..2/-
4/378
17.7.84
: 2
Criteria of a rational drug policy should therefore be discussed
in this perspective-and with the view that the drug policy should
be consistent with the-rational health policy.
It could be dis
cussed in the following aspects.
Medico-scientific aspect
Thi trend and pattern of drug production (including import) have
been fairly revelled in the report of the Hathi Committee (1975)
as well is in a few subsequent works.
It has been conclusively
shown that drug production does not bear a parallel or reciprocal
relationship with the disease pattern or need of the country.
Neither it is always governed by the medico-scientific justifica
tion. ■ Medico-scientific justification should act as a primary
criteria and on this basis the following issues come up for cons ideration:
E f ficacy:
a drug must be a drug according tp ..its accepted
definition.
It must have a tractable
or measur
able role to play in protecting or maintaining or
restoring health.
In India, various substances are consumed as drugs in the prevail
ing different systems of therapy or even belonging to no system at
all.
In order to obtain license as a drug, a substance must fultil
all the universal scientific criteria - no system of therapy
could claim any special criterion for itself.
Be-nef it/Risk ratio should be a guiding principle for the
introduction or continuance of a substance as drug.
Lower
the ratio, restricted be. its indications.
Pharrnakokinetics,
Toxicity and side-effects are the f ictors to consider.
Storage requirements and Shelf-life:
These considerations
hitherto neglected, should be accorded their due importance
■and relevance.
Bioavailability:
The issue of Bioavailability does not pose
a practical problem and should therefore, be ignored at
present.
Socio-political aspect
Priorities in the selection of drags for production, distribution
and research shoul d be governed by socio-political realities and
necessities.
Since the drug industry and trade are dominated by
the TNCs -and regulated by the trends of the international capitalist
economy, a people-oriented drug policy will necessarily come into
conflict with the prevailing trend.
Hence,- a radical change in the
drug policy hardly appears to be an achievable object.
The criteria
dn this reg-rd may therefore dwell on the considerations of opera
tive feasibility.
Priority druqs:
It hardly needs emphasis that we have to set up
oriorities for the production of drugs according to the prevailing
disease pattern i.e., drugs to combat communicable and nutritional
diseases should claim priority.
The prevailing much used term
'essential drugs' appears to raise some confusion.
Since every drug
has its distinct efficacious role to play, each one may be viewed
is essential - some to many and some to only few.
Hence, it is
suggested that the idea behind the term 'essential drugs' may be
oettcr served by using the words "priority drugs".
3/-
.'-4/378
±n any case, priority drugs ought to he made available to all and
such a situation could hardly be achieved without total control
by che state.
Presently, it appears that there is hardly any real
hindrance for the Govt, of India to exercise such control but the
fact remains that the Govt, does not exercise it and the parliament:
has failed to take the Govt, to task for its dereliction of duty.
The experience of Sreelanka and Bangladesh has shown that by pur
poseful and active intervention, the state could achieve effective
and significant change in the chaotic drag situation and this -'s
particularly so in respect of priority drugs. Hence, for the ade
quate production of priority drugs, an effective mechanism for
exercising state control should be formulated and implemented.
Distribution:
State health care service.including drug distribu
tion should cover geographically the whole country and be delivered
free to only indigent people (living below a predetermined income
level) .
Like health care, other categories of population will have
buy drugs.
Leois lotion:
A National Drug Authority as recommended by the
Hathi Committee be established alongwith the inclusion of repre
sentatives of the social action groups on .Drug.
The powers and
functions of Drug Control Authority should be subordinated to the
NDA.
NDA should be empowered to impose control over prescribing
norms of the medical profession and should time to time issue
guidelines in this regard.
NDA should ensure that all scientific
and legislative informations on drugs are circulated to the medical
profession. NDA should also recommended to the Govt, on the legis
lation in the matter of promotion, packaging, public education
regarding drugs.
Indigenous drugs:
A distinct effort for the promotion of indigenous
drugs in the name of old glorious Indian tradition is noticeable and
should be looked into objectively and not emotionally.
All indi
genous drugs ought no fulfil the medico-scientific criteria and be
evaluated on the economic basis (see below).
Research & Development:
Priority on R & D should be guided by the
disease pattern and economic criteria.
Foremost priority should be
accorded to those diseases which are real problems of Indian people
but have no ideal drug i.e., effective, safe, cheap etc.
R & D of
indigenous drugs only for the sake of being indigenous have little
claim on priority unless it is economically more cost-effective
in comparison to available drugs.
rie a 1th Educ at ion:
Education of the public on all aspects particular
ly hazards and cost of drugs should be an integral part of Health
Education.
Medical Education:
The conversion of the GENERIC education of the
medical students to the BRAND education after graduation should be
resisted.
Informations on all aspects of drugs should be made avail
able to students.
NDA should undertake the responsibility of con
tinuing education of medical practitioners on advanced information
of drugs.
Drue, Culture:
Priority of health education should be
towards an attack on the prevailing drug culture.
pointed
Con-uric name (I.N.N.) :
Should be compulsorily introduced with
suitabl e legislation.
A system be devised to introduce non
proprietory names for the combination drugs.
....4/-
I-4/378
;/17.7.84
Ec on o.-. ic as ~oec t
Technology:
Technological reality should be assessed and
necessary import of technology should be vigorously pressed
if necessary, in r-spect of priority drugs.
An international
campaign shouldd be launched for the dissemination of technology
know-how through international bodies.
Production:
Definite production quota for priority drugs should
be imposed on individual producer.
Pricc:
In respect of priority drugs,- prices’should be uniform
without any mark up.
All taxes should be abolished.
All import
should be channelised through state agency.
Non-priority drugs:
should be licensed in a separate category and
so designated in. the packaging.
High taxation is desirable.
Othur Issues -
drugs banned in other countries:
should be banned in India till
its fulfilling the above criteria is reevaluated and reestablished
Implementation:
with.
should be initiated in the state sector to begin
Nationalisation:
If other methods fail, Drug industry and wholesa
trade should be nationalised.
VoJuntry Health Association of India,
C-14, Community Centre,
Safdarjung Development Area,
New Delhi-110016.
3-4/378
k/lo/7/84
Background paper for
DRUG ACTION NETWORK (DAN)
Core'Group.Meet, Wardha,
July 30-31, 1984
From: Dr. W.V. Rane
Dr. A.R. Patwardhan
DRUG PRICIMG AND PRICING POLICY
Introduction, and acceptance of generic names would solve all
the problems of pricing (barring a few accepted combinations).
But as long as generic names cannot be accepted, it is advissabie to adopt the stop-gap measures suggested hereunder.
Present system of indirect promotion of drugs to a common man
through the medical profession and eventual selling through
series of middlemen (distributors, stockists and retailers)
brings direct and indirect burden on the consumer. The govern
ment, in order to safeguard the interest of the common man has
introduced many directives - which have been ill-utilised by
the pharmaceutical industry.
1.
CATEGORISATION OF DRUGS:
In order to make essential drugs
available at reasonabl e roates, the government formed
four categories of drugs.
Category I drugs were taken as
essential and a mark-up of 40% was allowed thereon.
In
order to cover the losses if any, the government allowed
the manufacturers to charge any price on category III f.-. IV
drugs.
The END RESULT: the essential drugs are not avail
able. and that the tonics, multivitamins, cough syrups,
hormone combinations, anabolic steroids, ensyme etc.
products are heavily promoted and within a span of one
year the prices of these products have doubled or trebled
and that the sales have increased.
This is mainly due to
indirect promotion of 1not-so-essential' drugs through the
medical profession. More money and imagination is expended
by the pharmaceutical companies in promoting these pro
ducts. Eventual loser is the end consumer.
How can this be remedied?:
a)
fixing leader prices of not-so-essential (Category III
IV) products by grouping them irrespective
of various combinations.
These groups can be:
Multivitamins, enzymes steroids, cough syrups etc. etc.
b)
Imposing price control on all categories of products
and even allowing higher mark-up (than the present) on
group I & II products.
c)
Imposing certain restrictions' on sales promotion of
not-so-essential products viz no samples, no presents,
no fancy packings, no lay-press advertisements and no
schemes or bonus offers.
d)
Banning the visual aids-charts and strictly checking
the medical literature.
cc"J!wiunity italth cat
47 /First Floor)St. Marks fioaci
BA:-'GAxOaH-560 001
-4/378
/16/7/84
:
2.
2
:
DRUG PRICE CONTROL AUTHORITY
Drug Price Control Authority .was created to control prices.
This authority should review the prices every two ye -rs .
No special facility should be given.to any section of the
industry.
A few examples of gross discrepancies are given
hereunder:
C ompos it ion/
tablet
Product
A.
Cost
100 tablet?
Rate
CorbutyK Roussel)
Dextrooropoxyphene 65mg.
Paracetamol 650mg.
2.15/6
35.33
Norgesic(Cipla)
Dextropropoxyphene
32.5
Paracetamol 325mg.
3.11/10
31.10
How can one justify virtually the same price for exact
ly half the contents?
Proxyvon
(Wockhardt)
Dextropropoxyohene
65mg.
Paracetamol 400mg. 4.68/6
D i azepam
2mg.
78.00
What justification there can be for reducing paraceta
mol by 250 mg. and aciding diazepam 2 mg. and taking
more than double the ruling (of Corbutyl) prices.
B.
Mictopyrin
(N icholas)
Acetyl salicilic acid 350mcj« „ 7ft/in
20mg. °-78/10
Caffein
Micropyrin-C
Aspirin
Vit mi in C
350mg.
25 mg.0.54/4
7.80
13.50
Merely dropping 20 mg. caffein and adding 25 mg. Vitamin C
has doubled the prices
C.
Essidrex
(Ciba-Geigy)
Hydrochlor
thiazide
Arkamin-H
(Unichem)
Clonidine Hcl.100mg.5j
Hydrochlorr
thaizine
20mg.
Arkamin
C Ion id in e
Hcl.
100mg.4.20/10
l.O.
Hydrochlorthiaz ine
20mg.
Now when 25 mg. tablet of
available for less than 5
ing and packing included)
chlorthiazide enabled the
for the s ame.
25mg.
0.46/10
n
4.60
5 2.80
42.00
1.08/10 10.80
hydrochlorthiazide (essidrex) is
paise a tablets (cost of tablet
mere adding of 20 mg. hydro
manufacturers to charge 11 paise
There are many such instances and these should be
reviewed by the Drug Price Control Authorities.
1-4/378
V16/ 7/ 84
3.
MIDDLEMEN AND COMMISSION
The unions ’and association's of chemists & druggists have
become powerful and they are now demanding 10% wholesellers
commission and 20% retailers commission on new introductions
Gradually they will make these terms applicable to existing
products as well which now give 5%-wholesale and 12.5%
retail commission.
The ire present demand has naturally
increased the direct burden of 12.5% and indirect burden by
13.74%. This increase is due to increased excise duty and
sales tax on increased prices.
Take for example a product
presently sold for Rs. 100/- (i.e. maximum selling rate).
This price will attract roughl y Rs.13.00 excise duty and
Rs.4.00 as sales tax.
For this price the manufacturer gets a minimum price (i.e.
realisabl e price) of.Rs.84.65.
Now for getting the same
realisabl e price of Rs.84.65, a new price structure will bo
as follows:
Trade rate
..
..
..
Rs.93.11
Maximum selling rate
..
..
Rs. 111.74
which otherwise means that the end consumer has paid 13%
excise duty on increased price of Rs. 11.74 and he has also
paid 4% sales tax on this increased price.
That otherwise
means that by this new method, he is paying Rs.lJ.74
additional to the middle men-Chemists etc. and Rs. 1.53 addi
tional excise duty to the central government and Rs.0.45
additional sales tax to the state government.
4.
BENEFIT TO DMALL-SCaLE INDUSTRY
Benefit of price etc. offered to small scale sector has
been ill-used by the multinationals and big Indian firms..
Normally a multinations is not allowed to market a new
product or its combination - and even if marketted, it's
price is controlled by the. government.
In order to bye
pass these difficulties, the manufacturers have created
their own subsidiaries as small-scale industries and are
taking maximum advantage of the benefits offered.
In most
of these cases the f incy products
been marketted or that
new combinations of old products have been introduced at
fancy prices.
For example
:
Waite r-Bushnel
Metakelfin
Rs. 5.47/8
Chymoral forte
20.58/12 '
Amclox
10.25/4
Rs. 2 73.50/100
171.50/100
. 256..30/100
Martel-Hammer, Montari, Jagson-Pal, Dolphin, .Full Ford,
Blue-Cross are some such firms.
Normally the products manufactured by a small-scale indus
try are marketted by the parent multinational. The govern
ment should now allow the multinationals and other big
firms to market the products of such small firms.
Before
making any of their new product available to their own sub
sidiary, it should be made available- to any other smallscale industry.
The rates of all such new products should
be controlled and fixed by the government.
5.
ATLEAST ESSENTIAL DRUGS TO BE MADE AVAILABLE IN GENERIC
NAMES
Anti-leprotic, anti-malarial, anti-tuberculour, anti-biotics
anti-filarial, analgesic drugs atleast should be mark., teed
only in generic names and their prices reduced.
Once these
products are converted into generic forms, these will not
attract excise duty and the state governments can wave off
the sales tax.
The whole-sale and retail margins on these
generic products should be fixed only at 5% and 12.5% res
pectively.' .These measures will make these essential drugs
available at virtually'half the present prices.
Leader
price should be fixed and any addition of anything should
not enable to increase, or cross the leader price.
pharmaceuticals, erug policies and health cars
FIRST BIBILE MEMORIAL LECTURE BY DR. V.T.H. GUFARATNE,
REGIONAL DIRECTOR, WORLD HEALTH ORGANIZATION, REGIONAL
OFFICE FOR SOUTH-EAST ASIA, AT THE PERADENIYA UNIVERSITY
CAMPUS, SRI LANKA,
13 FEBRUARY 1979
q-".nrr.T’rTY I’TAtTH CS-?»
■ ,.5or)St. Marks Road
■
,;l ...
560 001
I am indeed honoured to deliver the Bibile Memorial.
Oration. The late Professor Senaka Bibile was a pioneer and a
visionary who devoted his life to revolutionizing and rationali
zing drug policies and drug manufacture in Sri Lanka, always
keeping in mind the common man as the beneficiary.
The rich,
multifaceted personality of Professor Bibile could not remain
confined to the technical field of psycho-active drugs and
their control, in which he unquestionably excelled. His clear,
humanist vision perceived the problem of drugs in its larger
health context: how to supply safe and efficacious drugs that
would meet the demands of the expanding health services.
To this
great son of Sri Lanka I wish to offer my humble tributes on
this occasion.
Professor Bibile's was a remarkable life.
Like those of
many great men in history, it was a hard and self-made life. He
had his share of trials and tribulations right from his days at
school, which he had to leave for want of suonort. Thanks,
however, to the assistance of some elders in Kandy, who even at
that stage perceived his potential and intelligence, he was able
to pursue his studies further. Later, he supported himself
through scholarships, one each year, living a severely austere
life within the limited resources available to him. He had a
brilliant career, winning first-class honours all the way- Tn
the final medical examination be won the Djunjisbaw Dadabhoy
Gold Medal in Medicine and the i-iockwood Gold Medal in Surgery.
s harp inte i.1 iEndowed with rare qualities of uead and heart
gence, a deep perception of society and its problems, and
an extraordinary sensitivity of mind right from his school
days - Professor Bibile shunned hypocracy and false values and
always strove hard to help his fellow men. His disillusionment
with the commercial values prevalent in me die al practice was
mainly responsible for his dedication to a career of research
and t eaching.
I had the pleasure of knowing this extraordinary
nan perso nally throughout my career.
T was always struck by
his since ri ty, his dedication to research, his deep insight into
the problems facing the countries Ln this part of the world,
his encyclopaedic knowledge of pharmaceuticals, traditional
medicine and many other fields, his multifaceted per so na li ty
above
his
with interest in music and photography ano,
for life.
The rational use of drugs ; md pharmaceuticals and their
vital role in health care program® es was a subject which occupied
T
a major part of Professor Bibtie's work during his lifetime.
would like to touch on some of the problems in this field as, in
my opinion, this would be the best way to pay tribute to this
remarkable scientist and humanist.
While drugs alone are not sufficient to provide adequate
health c .re, they do play an important role in protecting,
-2-
maintaining and restoring the health of people.
Tn recent years,
there has been a very great increase in the number of pharmaceut
ical products marketed, but with no proportionate improvement in
health.
The development of drugs in modern times has resulted in
the huge growth of multinational drug companies in the western
world.
These have been operating for the last few decades in
the developing countries and marketing products, unmindful of
the health needs and health priorities of these countries. The
nature and magnitude of the problem can be visualized if wo take
a hard look at some of the facts and figures.
The role that the commercial promotion of drugs, amongst
physicians and paramedical personnel plays in distorting t'-e
demand for pharmaceutical products in developing countries should
be noted. Using hig'"’ powered methods, like gifts, books, travel
to int.-ernationa1 congresses, and sometimes not even stooping at
the dishonest practice of the "kick-back", by which the prescribing
physician gets a percentag’ of the cost of what he proscribes,
some of t'-e multinational companies are playing havoc in the do
developing countries.
Pharmaceutica’ oromotors that visit
physicians and other hea.lt'- personnel, are in a ratio of 1 to 20
in Britain.
rjowover, in a developing country like Tanzania,
there are about 150 promoters for a total of 600 doctors, a
ratio of 1 to h.
According to a r.cent report by a British
Physician, working in Dar-es-Salam the amount spent by the
commercial companies in the promotion of drugs in Tanzania is
estimated at £ 1.07 mi1 lion per year. This amount is higher,
by more than 100 thousand pounds, than the total budget for
the faculty of Medicine I
The total value - at retail prices - of all pharmaceutical
products sold in the world during 1977 was 75 billion US dollars.
To comprehend this colossal figure, let me state that the amount
could buy well over half of all the rice, wheat and maize
produced in the world during that year.
The differences that exist in the effective economic
demand for pharmaceuticals as between the developed anl the
developing countries are startling.
For example, the Garman
Federal republic spends US B 53
4
*
per parson per year, Japan
spends US B“ 38.5, and the USA S 35-1, while Nigeria spends US S
1.20 per person per year, India 75 cents and Sri Lanka 58 cents.
And yet in many countries the drug budget still represents
a sizeable proportion of the tota1 health expenditure. Tn
developed countries it ranges from 10 to 30$ of the tota1 cost
of health care. But paradoxically in some developing countries
the percentages are much larger, reaching up to 30 to 50$ or
-3m°re , particularly on account of the promotional activities of
the manucafturing concerns which create a demand greater than
the actual needs.
In fact, the problem is considerably accentuated
in developing countries because of the launching of nation-wide
programmes for the eradication or control of communicable diseases
and the development of the primary health car; programme from the
grass-roots level covering large areas and populations. The
expenditure on drugs as a percentage of total l",ealth expenditure
in sore countries of the South-3ast Asia Region makes interesting
reading:
in Bangladesh it is 63-8$, in Nepal 44.3$, in Thailand
30.5%, in Burma 24-5$ and in India 18.8$. The figure for Sri
Lanka is, however, only 7
5$.
*
The
the efforts
co ntribut e
pr oduc tlon,
low figure in the case of Sri Lanka is a tribute to
to which Professor Bibile very significantly
by introducing a rational aooroach to the procurement,
supply and use of pharmaceutical products.
In the late fifties, Sri Lanka had, in common with other
countries, a bewildering variety of commercial brands of drugs
aggressively promoted by drug companies. On a request by the
Minister' of Health in 1959, Professor Bibile undertook the
challenging task of putting some order into the prevailing chaos.
He was appointe ’ as the Chairman of a newly formed Formulary
Committee.
The Ceylon Hospitals Formulary, the little red book
which was an indispensable guide for a practising physician, was
his handiwork. It also marked the beginning of a process of
rationalization of drugs and pharmaceuticals in Sri Lanka.
One of the ways adopted by Sri Lanka to reduce the drug
budget, while at the same time increasing the availability of
useful drugs to the community, was the devising of a sound system
of procurement in th3 Internationa1 market.
An outstanding
example of this is the procurement of diazepam, which could ba
obtained at one-fiftieth (2$) of its previous cost.
In the light of these considerations the need is, naturally
to utilize available resources optimally.
The pharmaceutical
industry operating in the country must therefore decide the
direction of its growth keeping in view the needs and aspirations
of large segments of its population. Tonics, most of which are
alcoholic preparations mostly outmoded wholly or partly, have
the sole merit of being palatable, and they are the money
spinners fox- the manufacturers. These form the major portion of
the turnover the product mix of the aharmaceutical organizations,
operating in develooing countries.
It is essential therefore to
monitor the pattern and spectrum of drugs manufactured and regulate
the range so as to servo the real social needs rather than the
artificially created market requirements.
Irrational combinations,
exaggerated claims and excessive dosage of vitamin preparations,
which is indeed a national waste, must all be adequately
scrutinized.
-4-
Drug prices have became a nightmare for the co'tnon man
in developing countries.
Tn purchasing drugs the patient does
not have the right to choose what suits his Docket, as is the
case with oth.-r commodities.
He has to submit meekly to the
purchase of drugs which the physician prescribes. High-powered
propaganda, backed by an army of representatives, often succeeds
in influencing the doctor to choose a particular drug. Besides,
in developing countries largj sections of the population depend
on the health facilities provided by governmental or semi-govern
mental institutions for medicare. Tf the prices of drugs are high
the drugs that could be procured within the limited budget of
the institutions concerned are bound to be severely restricted.
The State Pharmaceutical Corporation of Sri Lanka in its report
for 1976 has brought out interesting data on foreign exchange sa
savings to the tune of over B 112,000, on the purchase of just
ten items of drugs, as a result of careful "world-wide shopping"
as against the oId practive of purchasing it from the local
market. Tn India, the Ministry of Petroleum and Chemicals,
through its price review cell, proposed a reduction in the prices
of a large number of drugs, bringing about savings to the
community to the tune of over ,.ts. 200 minion. The international
pharmaceutical industry is heavily dominated by nearly a 100
largo multinational companies, holding the monopoly for a
number of bulk drugs which enter the formulations. Sven in
developed countries, there is serious concern over the excessive
cost incurred by the Irug industry in terms of excessive pro
motional costs, "me too" drug proliferation, high prices for
basic drugs, and a large amount of avoidable overheads brought
about by highly attractive packaging, etc.
It is, therefore,
important for the nations of ths third world to evolve a "nationa1
drug policy" to ensure the availability of effective and essentia1
drugs in sufficient quantities at economica1 prices.
Thanks to L’efforts of Senaka Bibile, Sri Lanka has, through the formation
of the State Pharmaceutical Corporation, given a lead to the rest
of the developing countries In this sphere.
Sri Lanka has sot
a model for the management of pharmaceuticals, Including the
rational nd economical use of drugs, thereby providing a viable
alternative to being at the mercy of the multinational companies.
While discussing the problem of drug costs an I prices it
may bo of interest to examine the impact of "brand names" on
medical practice and drug pricing.
T’-e practice of selling -’.rues
under "branl names", rather than generic names, is Unkel with
many complex aspects of the drug Industry such as drug patents,
irrationality in medical practice, corrupting influences on the
medical profession, irrational drug combinations, and the oxcossiv
use of ingredients in evar-proliferating multi-ingredient
preparations.
It is a rather strange phenomenon that whereas
a medical student learns the use of ’rugs under generic names, ho
quickly switches over to brand names in iro.’ica1 practice.
often felt that a practising physician prescribes a "branded"
drug without even bain; fully aware of the composition an ’ doses
of the ingredients that go into the formulation of the product.
-5-
M.any brand names ar? phonetically so similar that they tend to
produce confusion and chaos.
Digenol - Dianabol, Codogen Codopen, are typical examples. To bring about uniformity in
names, '/HO issues periodically a list of non-propriatary names
with recommendations that these ba adopted by national organizat
ions in order to privcnt sue1- confusion tn proscribing medicines.
Branded products have, in most countries, been found to ba much
more expansive than generic ones. A olain aspirin tablet is
invariably cheaper than many branded oroducts contain’ng aspirin.
Interestingly, the same drug is marketed under several brand
names by different companies and the price variations in these
products ranges from 10 to 100 per cent.
In the case of multi-ingredient preparations, the situation
is especially chaotic. Often, unnecessary and irrational for
mulations are marketed and the gullible physician and the poor
patient pre duped.
In fact, much of the dominance in the multi
national. field or t'-e organized sector of the industry is
through aggressively promoted branded products.
The Food and
Drug Administration of the United States of America appointed a
committee of experts of the National -research Council (NRC) and
the National Academy of Sciences (NAS) to evaluate the efficacy
of over 3000 preparations marketed in the U.S.A. Their report
brought out the fact that a large number of these have no proven
therapeutic value.
Some of them were directed to be withdrawn.
Many countries in the r/est have been advocating the abolition
of brand names. This itself, it is felt, may have sufficient
impact on orices ;Hich may =>3 a result come down substantially.
The Sri Lanka St”te Trading Corporat’on needs to bo
complimented on its careful an': sagacious approach in this reg”
Here most drugs are generically named while in the case of a fe
the brand names are permitted to be printed in half t’-e size of
generic names. One of the powerful arguments against abolis^in
brand names is the fear of deterioration in the quality of
drugs. But this is hardly tenable if there is effective qualit
control, as is being exercized in Sri Lanka through quality
control laboratories.
Intimately linked with this issue is the
problem of new drugs. Most new drugs are introduced to the
medical profession through advertisements unler brand names in
international journals.
When these are introduced for t'-e firs
time in a developing country, they ar? often excessively price--'
and are invariably prescribed under brand names/ Further, ther
is an almost universal and impulsive desire on the part of the
medical profession to prescribe "fashionable" now drugs in
preference to well tried, established and very often low priced
drugs. Most new drugs are not a real "breakthrough" but mere
chemical manipulations of airenly known ’rugs in common usage.
Such "me too" drugs have only marginal advantages over the
already existing remedies, but these advantages are magnified i
order to push up sales an 1 take advantage of human foibles. Th
popularity of such drugs in prescription often follows an
-6interesting pattern. Initially there is a rapid rise followed by a
sudden fall and thereafter a slow rise to a steady level. This
state of affairs is vividly seen in the case of drugs belonging to
non-steroidal anti-inflammatory compounds.
There is a very keen
race between the various pharmaceutica1 companies to put out an
anti-rheumatic, -;ith exaggerated claims for efficacy, while in fact
none 01 the "new drugs" has been proved to be significantly better
that the age-old aspirin in its efficacy. While most new drugs
are in the field of hypertension, ’iabetas, atherosclerosis,
antilipidaemics and tranquillizers, there are relatively few for
the treatment of diseases rampant in the tx-ooical countries . 'We
have no breakthrough in the treatment of filariasis or leishmania
sis. Rifampicin, a very potent drug, has no doubt made a signi
ficant contribution in the treatment of tuberculosis and leprosy.
But the drug is at present so heavily priced that its introduction
has harlly made an impact on the community hea1t^ care services
in our countries.
Most of the giant multinationals are spending large sums
of money on drug research, but their priorities are tota’ly out
of tune with the disease pattern in the developing countries.
While our problems are still largely the infectious diseases,
research in Western countries is mainly concentrated on cancer
chemotherapy, problems of affluence an' geriatrics.
Drug research has become extremely expensive.
Tt is not
possible for one single country in the developing world to provide
adequate funds for research in tropical diseases. Tt should,
however, be possible to organize collaborative research on Irug
development among the countries of t'-is region under the auspices
of the World Health Organ! ation, so that resources could be poo lo
an-1 meaningful in-depth research into priority problems of the
Region could be carried out.
The developments taking place in Sri Lanka had their echo
in other developing countries, which began to recognize more and
more the soda1 limensions of pharmaceuticals.
By introducing
rationality in their managsment, much more efficient use could be
made of the pnera1 ly limited financial resources available to the
health services, increasing, at the same time, the ability of the
poor to purchase essential drugs.
Thanks to Dr. Bibile's pioneering lork in Sri Lanka, the
developing countries woke up to the fact that drugs cannot be
viewed from a purely scientific or technical viewpoint.
They have
to be looked at in the wider perspective of health priorities,
and necessary steps taken for effectively extending health care
delivery to all the population.
WHO has, since its inception, been interests-1, in phar
maceuticals, because it fully realized their fundamental role in
preventing an ', curing disease an:1 in alleviating suffering.
Tt
must be admitted, howev.-r, that, until recently, the Organization’s
-7-
interest; centred mainly on the more usual technical and scientific
aspects of pharmaceuticals, such as the establishment of specifi
cations for the purity and potency of drugs, norms and standards
for the control of the ruaiity of oharmaco ut ica i s an 1 biological
products, the adoption of criteria fer testing the safety an4, effi
cacy of drugs to be used in hurans, etc.
Its publications such
as the International Pharmacopeia and the Cumulative Lists of
International Non-Proprietary Names, are tn everyday use.
The /orld Health Organization has, however, turned its
attention to the wider aspects of drug policies and management
as essential components of national healt'~ planning and Diagram
ming.
This, in no small measure, is due to the interest sparked
by Sri Lanka's pioneering effort. The 23 th ’Jor’i Health Assembly,
held in 1975, considered a comprehensive report of the DirectorGeneral which analysed the concept of a drug policy not only in
terms of its implications to the health sector but also from
a multi-sectoral approach, with proper attention to the role that
industry, trade an 1 finance necessarily have to play.
WHO's efforts in this field have since then continuously
increased, both at the global and the regional level . Thus, the
31st World Health Assembly, hold last year, considered as the
subject for its technica' dtscussions "National policies an’
practices in regard to medical products an! relate 1 international
problems".
At the regional level, it >as in March 1978 that
participants from eight of the ten countries of the South-East
Asia Region met in Colombo, at an inter-count ry seminar, to
discuss the topic of -*'rug
policies *
ni management.
A drug policy, especially in developing countries, cannot
be complete without considering traditional me licine.
Most of
the remedies of traditional medicine, esoecia’ly when it has
attained the status of a "system" such as Chinese, Ayurveda or
Unani, have a high consumer acceptance. They are very useful
in the treatment of common and self-limiting ailments, in psychoso
matic disorders and also in chronic diseases, in respect of wh'.c1’
modern medicine has as yet little to offer.
These remedies, mostly
of plant origin, in which people have longstanding experience an!
faith, represent an important resource for health car;, being g
generally inexpensive and made from locally available products.
Attention should be given to ensuring the preservation of this rich
cultural heritage of traditional medicine, whilst, at the same
time, promoting research into traditional preparations using
scientifically valid methods.
Such is the importance attache4 to
this subject by the countries of this region that the topic
"drug policies including traditional medicine" has been chosen for
the technical discussions to be held during the 32nd session of the
WHO Regional Committee for South-East Asia scheduled to take place
in New Delhi in September 1979•
A National Drug Policy should bo evolved in the context
•8the health policy of the developing countries. Broadly, the
drug policy may have to take into account several factors:
-
Essential ’rugs must be co rmo nsurate with the spectrum
of diseases.
-
Such drugs wi’1 have to be available in abundance to
meet the health needs of the people.
-
A careful 'watch will have to be kept on quality and
preventing adulteration and other related malpractices.
Self-reliance in the output of drugs shoul ' be aimed
at, so as to reduce dependence on imports.
Research and development should be encouraged.
Each country should select the priority pharmaceuticals
needed by its people.
There can be several criteria for the
selection of such drugs, including:
-
disease morbidity data,
diseases of public health significance,
Irugs having a single ingredient rather than combinations
(if at al’ fixed ratio combinations are included, tho
phar ma cologica1 an 1 chemical 'ata should justify such
a combination),
drugs that are economical, easy to manufacture in a
country an! non- pa to n ted.
Most developing countries have their national formularies but it
is necessary to remodel these to categorize drugs? as the most
essential for mass consumption, say in the national health
programme, mainly to bo used in primary ha t It b care by co munity
health workers (CHZs).
Such a list may not contain more than
20-30 drugs.
A second, more elaborate list shoul' include those
needed for patients referred for treatment to JI strict-lev el
hospitals, A larger list containing 200-400 ’rugs which have
been well tried and which also contains all the essential Iru "s
should be drawn up for use at the apex referral institutions.
A Wr’O Export Committee has male a selection of 177 essential
drugs together with 32 complimentary drugs "corresponding to
health needs, keeping in mind the situation of developing
countries".
This is only -a mode’ list and may have to be modifi
depending upon the needs of the country. Further, such a list
cannot be static.
Tt has to undergo changes depending upon heal
priorities an! epidemiological considerations on the one han ’ an
drug development on the other.
Such a list will naturally liffe
from country to country Jepeniing upon several factors sue’- as
disease prevalence, health infrastructure, financial resources
and the stage of development of the drug industry.
-9In the late fifties Sonaka Bibilo's attention was more
and more devoted to problems related to drug policy and
management.
His interest in the subject of controlling drug
abuse came at a time when the international community felt
the need to create a new Internationa1 drug control treaty,
in order to contain the abuse of the large number of newly intro
duced and potent psychotropic substances around the world.
T'->e increasing availability of psycho-active drugs of
many
nds - hallucinogens, barbiturates and amphetamines unfortunately gave rise to widespread abuse.
The abuse of
drugs is as old as mankind itself.
The Convention on Narcotic
Drugs ol 1961 aimed at protecting the health of people around
the world from drugs obtained primarily from plants, for example,
those obtained directly from the opium poppy, cannabis and coca
leaves as well ->s those synthetic derivatives of the same types
including morphine, heroin and cocaine.
Subsequently the
Convention on Psychotropic Substances came into existence in 197^
*
The continuing tendency toward increasing abuse of
psychotropic substances is noted in most regions of the world,
particularly witi-> regard to the abuse of amphetamine-type drugs,
sedative hypnotics such as methaqualone, barbiturates such as
secobarbital, tranquillizers such as benzodiazepine and halluci
nogens such is phencyclidine and lefetamine.
At present syste
matic information is being gathered by the ’ZHJ Division of Mental
Health on several of these substances in order to determine
whe ther current international controls are satisfactory according
to their present scheduling under the Psychotropic Convention.
The countries of the South-Dast Asia Region are deter
mined, in the spirit of the Convention on Psychotropic Substances,
to see that these substances are available for medical use
whenever needed, but at the same time to prevent their abuse
primarily through an ill informed medical profession.
The late
Professor Bibile contributed in no small measure to these
developments.
'.Zhile 'is contributions to his native land have
bean remarkable, his work extended far beyond the shores of
Sri Lanka. Outstanding amongst his overseas activities was his
work connected with the Caribbean pharmaceutical projects just
before he died.
In 1976, following a meeting of the group of experts on
pharmaceuticals held in Georgetown, the Secretariat of the Action
Project for Economic Co-operation among Non-Aligned and other
Developing Countries initiated preliminary survey in the Carib
bean with the co-operation of the United Nations Conference for
Trade and Development (UNCTAD) and the Caribbean Community
Secretariat. Professor Bibile came to Georgetown in August 1977
to provide the UNCTAD input for t'^ts project.
He took part it.
the joint mission to ten countries in the Caribbean to collect
-10--
information required for initiating concerted action by those
countries in the field of drug policy, production, procurement
and distribution of pharmaceuticals.
The significance of Professor Bibile’s enormous contribut
ion becomes most clear when it is realized that the re co nmondatior.s of the Pharmaceutical Testing of Experts in Georgetown in
July 197 6 were to a great extent based on his own successful
experiment as the Chairman of the State Pharmaceuti ca1 Corporation
in Sri Lanka. The recommendations of this meeting were later
endorsed as Resolution 25 by the summit meeting of the Heads of
State or Government of the Hon-Aligned Countries at Colombo in
August 1976.
The Caribbean oharmaceut tea1 study was the first
attempt to implement 'his resolution.
During the tour of Caribbeans, which often involved tra
travelling every 3 or 5 days, Professor Bibile was not only the
main guiding force but worked almost every evening and even
some week-ends documenting the observations of the team. His
personal contribution was t'-e collection of information from
the public sector.
Then the Mission returned to Georgetown on 22 September,
in order to complete the report by the 11 October 1977 » Professor
Bibile set himself tough schedule of ';ork, involving evenings
and starting work very early in the morning.
During this period,
he was also very anxious t’~at he should finish tho work on time.
Exactly a week after '->i3 return to Georgetown, on 29 September
1977 j Professor Bibile died.
The report was finally completed
on the principles established by him.
Such ./as the remarkable life and work of Professor Senaka
Bibile - a life of great devotion and dedication to the cause
that he took up as his mission.
Professor Bibile’s contribution to the field of science
was no doubt significant. But equally relevant were his human
qualities. His zest for living and enthusiasm, his kindness and
generosity, warmth and affection for peoole, his gaiety and his
loud laugh, his sympathy and attention in times of illness, are
too uli known.
T would like to say in conclusion that the inspiring
contributions made by Professor Senaka Bibile for ensure ng the
availability of effective drugs to ail in need, and a rational
use will certainly he 1 o us significantly in our quest towards
our avowed goal of he?ltb for all by the year 2000.
E-4/378(c) LCD&RT
: 17,7. '84
TOWARDS A RATIONAL DRUG POLICY IM INDIA
Anant Phadko.
Even a cursory glance >at the existing ‘drug-situation in India would
reveal that the production, distribution, use and monitoring of drugs in
India is today a far cry from a Rational Drug Policy. All' the aspects
of existing drug-policy need to be completely overhauled in order to
effect'a Rational Drug Policy; Such a change would involve amongst other
things, nationalization of all. the major drug companies along with a
social control over the national-ized sector.
But such a step cannot
be expected in immediate future because of the low level of awareness
about the necessity of a Rational Drug Policy amongst medical personnel,
politicians, government officials and the common people; and because
of the weakness of the people’s Movement in general. Hence only the
following intermediate measures are to be demanded from the Government.
These measures do not constitute a full-fledged Rational Drug Policy
in India but are steps in the right direction. We demand from the
Government the following:-
Setting up of a Drug Review Committee to assist the Drug Controller
of India. It would scrutinize all the drugs currently marketed in
India, on the basis of principles outlined in the "Essentials of a
Rational Drug Policy". The recommendation of this committee to be
implemented without delay.
The Committee would be ..a permanent.body and would review the
drug policy every year in the light of new information on older drugs
and invention of new drugs. No drug in India can be produced or
marketed in Indic unless approved by this committee. Similar
scrutiny of non-allopathic drugs to be carried out on the basis pf
proven efficacy and safety in research.
The Drug Review Committee to prepare a Graded Essential Drug List
as. outlined earlier and the Government should make it mandatory on
all drug companies to produce drugs amongst this list only. All
other drugs recommended in standard text-books would be considered
as non-priority drugs in India. A separate list of such non-priority
drugs shall be prepared by the Drug Review Committee and drug
companies would be required to take permission to produce or market
any drug from this list.
Only those non-allopathic drugs which have been proved to be
effective and safe in research are to be allowed to be produced for
general use.
The Public Sector should play a leading role in the manufacture
of life-saving drugs and drugs used in the National Health Programmes
(for example - Tuberculosis Control Programme). It is the respon
sibility of the Government to ensure adequate supply of drugs through
increased production and imports. Those private companies which
refuse to continue the existing level of production of drugs listed
in the Graded Essential .Drugs List,, should be nationalized by the
Government-to prevent fall in the production of these drugs and the
consequent shortages.
2/
CALTH CELL
1.
E-4/378(c)LCT & AT
V RAI:pt:17.7.'84
2
3.
No technology transfer agreement shall be legal and binding which
contains restrictive practices, disproportionate and unnecessary use
of imported intermediatorios or obsolete technologies qr unfair
arrangements with respect to prices, payments or repatriation of
profits.
4.
Imports of drugs to be governed by the same Graded Essential Drug
List and'conducted only through the public sector agencies in a
rational, planned manner and through competitive world-wide tenders
to reduce cost.
5.
The Drug Price Control Order of 1979 should be extended to all drugs.
However, the existing categories in the DPCO to bo abolished and a
uniform mark up to be al 1 owed on all types of drugs so that pre-tax
profit rate of a maximum 15$ is assured. The packaging, advertising
and other overhead expenses should be standardised.
6.
Abolition of excise duty and salcs-tax on drugs commonly required
mainly by the poor people - antibiotics, antimicrobials, anti-helmi
nthic drugs, drugs used in the treatment of scabies, ringworm
infestation. Exemption to be granted also to vaccines of all types,
and drugs used in the National Health Programmes.
7.
Marketing of drugs only under generic name. The company's name
can be mentioned on the label in bracket. For example - Ferrous
Sulfate (Glaxo). But the generic name should be at least equally
prominently and clearly printed on the label as the company's name.
8.
It shall be the primary responsibility of the manufacturer to ensure
the quality of drug products. However, it shall be the statutoryresponsibility of the Drug Control Authorities to monitor the
standards and ensure a minimum uniform level of government control.
Consequently, the government shall take all necessary measures to
enable the Drug Control Authorities to function in an effective
manner and discharge the statutory duties cast upon them.
9.
It shall be the statutory duty of the drug control authorities to '
inform health personnel and consumers of the essential drugs lists,
policies, categories or brands of drugs banned for manufacture or
sale, ttrough publication in the national newspapers, magazines,
medical journals with adequate‘explanations and
details.
10.
The marketing code drawn up by HAI (Health Action International)
should form the basis for a National Code for Marketing Practices,
This should be accepted by our government and should bo suitably
implemented through legislation.
11.
Adoption of the marketing code prepared by the Working 'Group
on the Formulation of the National Code on the marketing of Breast
Milk Substitutes.
12.
The Drug Review Committee should also scrutinize all the over-thecounter drugs md only those drugs which are fully scientifically
justified to to sold- as over-the-counter should be allowed to bo
sold in such z. manner. No vitamin preparation he allowed to be
advertised in the lay press and all advertisements in the lay press
should be preiensored to prevent any misleading of lay people.
3/
B-4/378(c)LCD & RT
VHAr:pt:17.7.'84"
13.
/abolition of wholesale stockists and distributors - the unnecessary
profitmaking middlemen. Establishment of National Corporation for
the distribution of drugs and Pharmaceuticals to the retailers.
This Corporation to work on no loss no profit basis.
14.
Making mandatory for doctors to keep proper clinical records.
The records arc open to scrutiny and doctors answerable to a
regional bureau of medical experts which would investigate
allegations of misuse of drugs by doctors.
15.
Punishment to unauthorised persons or to chemists for giving
drugs without a doctor's prescription.
16a
Inclusion in medical education a study of Rational Drug Policy
and the current status, measures in India.
17.
Compulsory continuing education of all doctors and medical personnel
through journals and refresher courses.
18.
Education of the lay-people about over-the-counter drugs and about
misuse of drugs.
19•
AH the above measures
non-allopathic drugs.
should also be applied in case of all
Position: 88 (37 views)