RF_WH_.6_A_SUDHA.pdf
Media
- extracted text
-
RF_WH_6_A_SUDHA
o
53080-darfoad
ssdreertd
age3ris&
V
83
/■Jgijt ?±)^ei>
vBBFsukshema
Impnwed MMmwl Newborn & Child Health
'•Witl.F<i>if)« WM fhne
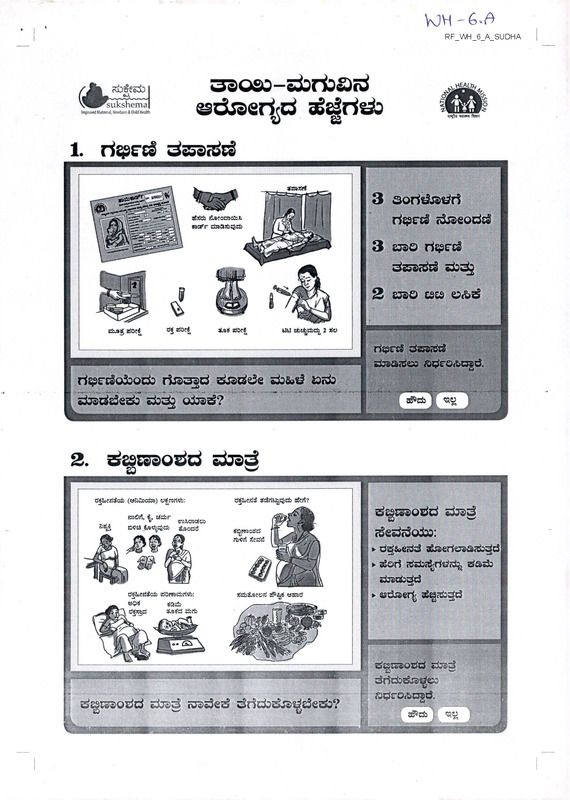
1.
acsstri
3 sorfc&a^ri
ri^cES rijaeods^
aiddb rJjseorootoX
iran^r dsaCBdjdicb
3 era©
dcSsrdEi 5±i^2
2 essQ ES£S
dbjad, doeS
d-S dOe<
WU tisidbdb 2 do
qtatf doe«
■
f^e-$ dasa^d
djaadoo ade’O^ogd.
ri^E-dofrodD risasred s&adde dowsi iodo
.-,■■■•.•.
--' ■ • *,
■
■
-:«»
- ■
•
.
..
--
-
■•
.A.
■: ■
• ■.
...
daaddeds dsda
odad?
—o
I
I' rz; i
.
k taw
~
2. desEsao^d
drad
ED
o
d&oegteoi) (WsD^ocdsa) e^rari^:
ssOrt, &, dzhr
. _,
*>
tnxxMtVi
o?a„M$S0di ijsodd
A^JL
djrkfwi d^rtt^^db aSeri?
d^Eaaodd doad
etonraodd
rb<?rt jJede!
I deddo&D:
y^jp
| ► d^&ecdd a»raeriCTa<&3d
► aSori
&,»
1\
d^SoesJSci dOrradbriVo:
t5$?$
tfasb
dJrX^S
d/sjrd dart
djadodd
► asdsaer^ d^z&dd
afch&MOKl a?5^ warod
(tswi
-
UZ
'\’h
■
■
■.
d^Eaaodd doad roaded dridodia^dedo?
a^sraotfd dasd
tv dtictos&a^eo
| fJ^E:'o*n§d-
L -IT^I
1
' r
x£>3e&±i
^B^svXshema
Impwred Mjtwiial, Newbwn & Child Heakh
saoeo-cabrtoad
esdraertd
de^rida
W
£3
A
<i«<lii ki<«i Pm
3. rt^racdo^ ©sssodod ^desr&o
T
CO
ri^racddod 9
aoiida ©djod
w
q±05DdO W&3
3dcSjaesg
die)^ esdQ. de
ssddodes doda
sadad ©zsaodd
eadsaridd^
n
e>3c wg>o^
sSrfe^f*,
grTIVi
vo^csUd
4)^
enoO s&te3,
3jsoc33
riada@& d|d$©od
dJsd
23&dca
-x>
dddad&^deda
ri^rE3o±>®
dodaejdaci 16
©siraoda sss^rida
oiros^s^?
ssd^ iuadeda
draddedo?
3eg
□SjSt^.djs’Cs^)
e>z$9 31fi)^r
coOrt eSjsecJ)
3esd—’ fcsU
U-®
o3js>c£)0&o?3
oTOTjcScdo
TJS,«3 .
r/
*?»
osjacScCood
djs^d M
olfcc&o&od
33
f y^n/c^
S^OeS
<3Od <53:55)^
'W
”
J
'
[ spssaobd O5&®rtecto
.
atoerto
' I
ffii?£ra*a®tf.
, a^c* I
'
I
B
«*#
fl
aaoeo-dsrtiad
ssdraerird
ddrids
V
S3
vSB^iSshema
Improved MiternH Newborn & Child Health
«i<wi ft»w
4. a^odd &dd
Q
t»Jbdrt<#jS>l 4rtoct»crfa^^)d:
4 DOS 5 OSO
«as»C
t>4 1^4S
<U^
saoafd
fiil
^®*
C&»dt330t*
eSortrt c-iCnci
tfcrbart tJewci
irtOcfc^cfc
crassrd ^ddnL
cftal’Jd
£j»d:5e irtocfcSe*
•ms,*'
77?
atortrt w^S/$
aSJjjrtcart
^cb
cU^AtW *
K&o&ad <vd3
a^Jo±^n»rt cirf
.twaijiVes ui^cb
c&aas&as&aera.
aSOrtrt a&»;7t>c»rf fRsJ 2 ad
ero«=ijt»<Jw dibrWfi, ucs^tSefc
I11
s^odd
itsIp
atori Addri sdE-o&cjad.
aSoriri #<3e<&
siraa^geSedo?
II ||. I.
\
^c^'T w
5. cddsso cfi®©^risb
-’< -- ./,;?3TSSs:
--S >,,',
J- '
oiofsseSrte*
urtA dssSoa
cdsaro o&aeeicSrisi)
3c£)«^ca> ofcbnsf),
de otoescSridcktj
ddo±o«^ct
«
bI
I
ddsao
f
■ ■
. ' '
2E
'
f
.
.
.
.I.
^©■scd&od &rfc>zto^3>
ero^Gtoeriri^ecio?
V
'
. •‘'*'-:>;,WMig*
■
..
.
■ '
\.. '/
■I
e±k£) I
f;.:
^25 •
■^•.:..«W.
ddda^a^
sdE-o&ngd.
'
- a'....... .... —------- ' ’ • : M
............ ^g. W
h
mi? j
a®Qe3-5±>rtoa<d
ssdraert<d
sgdds^
V
S3
^l^sukshema
Improved Mottroal, Httvbcm & Child Health
4®Sf
<!'<)« «R«4 faVH
sdfdsd
6.
ddd ddaoiades
VsJa^
d^a/d^yxi:
CB£eS0S»tf,
dada doddd 48
rtodri^fg oiradde
<
d>t>£c&,i acda
ucb^jda
daaoddd&adeadada.
sade^n dododaad
dodd 2 od ssddcdaes
\ t Az —y
12 rtodnostv.'j ddtf dort
eictrad dix^d
enasodadededa.
4Vqf rfjidco dartadcj id,
▼
wtddc
sada^ds
<doddd 48 rfodrid<g
g&dd <&±)o±)d<g
■dodo eadod djaddarfeto o&as^? ®dd, <aadeda daaddeda?
-
ss^d agori siJe>a*d
riodd 2 aad erusoisos
a^FO&ngd.
................
•
.............
7. &C3
dexadsA
aroear^jiad
don
drsdoiefcj sn>tx
fcBdad dad. j
^i^daadat^ds <
sli»du Arad
awifej dartadrt
fcaxdfd
Cd asaeaa esd^d.
6 soridddr? ad
da^m^od©
•ad^ dartadrt
aspoarfsiadl^
sgjBudsa ssct £)^.
11
I -vU-a
25i>da^^aaaart dartadda^ did dacA&iraVdefe
asaex saw
u e^edecdsa
6 aoritfddrt
ddaraax
das^ asda^da
ssteoa seatoari 6
©otrfeid^ ridwadw;
PHE
' ’TT
' Jt’-.
MBI
B
, &dca&ngd.
' adasaeaa aedasaad ddari riaaaddeerad 6
»a
| ©oddda odaa^s^?
Vt-sat-
.
aKiME"""
I
■ I
33Q£o-e±>rtoa<d
©draeried
dddds
U
S3
__
^l^sukshema
Improved Metemjl, Newbern & Child Health
A
eisfla wwfl then
essrao&d ©dcari^o
8. cdsdezsd
»3e t$ea
ziradrsado,
s^s)b8><S«be)Od353ari2»d
24 rtotfofcO
6 xJo^o^ tf&ds dxra^
dtoefcaoart
e^eari^r^j rbdoa*
essd
dz^ eszsa&aa.
elc Z3e)O3
:
eatortsb
d^e^de«b^d3
dusd^S <3£icS «3td3S$dj
CO4><t0
eruaaiiaoQcbd)^
deaod dsaSKsb t!o333rt)S$d3
■
■■
■
■
■
‘Etdo^db fc^da
ieradad ssfe
£j saw.
ddsr sdtfd erbE^ds
jbaoidc
^ebs^ds,
■
djai^b uosjartas^db/
wcbdjds
'acbdjcb
dderad Szbades
dodo adod
19 eszsaodd
ssz^ddo odads^?
®d^ <oad<g<&
adaddeds?
voiiuaud i/aodd
wsod alresj
^sraabci e^eartecte
dedddoa rt^rtvart^d)
£2<ggri atojertoD
■
B
^©<bC5g^.
fe • 27 -’.
L^£.
Mi -
Bum
..V^WrW^’ r
•
■
ssGao-edrisad
ssdraedd
ddrisd
6
S3
(JL---**---
VimiBr sukshema
Improved Maternal Newbern & Child Health
ri’tfta wrrw fhw
9. cdsdesad
dartadrt^ did
dbonx
iC, ics^rtfrt
aded*. arattasdda
aSji^b tdCrt
ara^dda
dartd*. uijctocd
*3 dWAarf!*
Kdasi fcsadjbj 7 art
xajrtdssajJrf
Dkjodod
e»d«± esdj^
ga zbrbaid
a»a^,
@7s>a^a<±^d2 aed
essTOErae.
(d
*^dfid rtodoSci
OXS araizbsi'Cb
X* U
ds&rad '&Ebad
| ©di^oiia 7 ©osirisid^
I rtebsdsa sdreAagd.
I ddarad adsazd esd(d aedoaad <u®d)
•
7 esodrido oiras^4?
I
IO. S3c)£3Q3O^
ro
••
-•■-*•
■
••
‘
•
•
•
■
--•.■-
•
-
■
•
-»
i
^£3F&0
dru gjasaoaoi)
g3ae<d oe? dsaeriri^
e^>
wxcreud tfjjodd
d^dodd. gs^ridzd^
ddcs rfc)dja&
clrsc^otood
ts^S d^ ^d
ied,
idf&wd)
dxiDdg truid
sSjdjdes esad
tag Etosssrtditd
rfjsd
rtdzjsrbd)^
• :
ddoddeda.
W z&ra^&sed)
I sissaedbd e^tarisdeg
:;rt:S‘S;iSS
| gssEsoacxt^ dods adod 11 essssodd
| ds^rids odaddr? &>dE| roadeds s&addeda?
Cxkt ....^.iBBaegat.
..-. rrwi
| ,e$^r? dsaeriex)
■<?
**
F-
ssoec-Etorfcad
esdraertrd
ggddsd
U
S3
<Jn
^I^suksherna
Improved M»temH Newbern & Child Health
A
wr<wi fttn
11.
aS^eesoisae £>&dsjaoeori,
es&& o&dd^aesari
dosaaddoa6 o&ddjadri
‘©&drida
esedd dsa ddd'.
^(&«3aFQ&)
rto&scot&so
Xa
'I
<iJc>Q£Od«±^
f>
I
a^ejjUFE6-^
'
-
. ..,.
.■,„
. *,
--iMk
-■••■
— <*.' —
■••>.•
•• -v-..X.V- *,
:
•
7
n)^ro<&c3gd.
I odsd odad draeririsr? oiraaari
gj| .. . .
’
..
...-.-■^
I asadddedo?
3a5
Q&de&c^
12.
vo^ti BazaSiatdO^
dado dartOTta
adolfjoi drtert
«tbs^ 3 ddrti eoad
' ^da^ddarta
cd&rte rddasS
aec&Drt:
s£r
SSS^OiS iUSOW
iraodjjedjf
jotoJjo &U3CW
c&MeaSrte
4<tofc
/
©oddades.
rioades a^ssd®
C8
alraeddrtsb
dsbot-a.
Wd3*-U CTioo—
/
I s S • S0 <
do^d ddod
SJiOibC &UJOW
©o^dades.
otafdFSrteb
i1
$£&
drx fct&owd tfrso
;
dowoa doa<£a oSaaeesdodad^ odad
i esdda&djaddeda?
_____
. . .:
.
I.......... -.7,
i£t3»w odeas
®si=la<’u^sa^e»
fe
ffitfco&rod.
B^WwiL .
"" *
J
2
I-
33Qe3-5±irba<d
/Jh ^ee±)
__ _
ssdraerird
v
^■^sukshema
Imptored Maternal Xwborn ft Child Health
*
as
<i>sJlq kiwi Pith
: dad wzpaod zbWaou £eo§,d doddd dozio
: dortrt daai dodaO dsad dz^djd^Ood doodad sarVa
erodes
Q
doagprar
ddoiodjda
: rtzprf^, darao£ daztra w^oi ^Wso’d^da
erode&d rtso^
zjs?dwdjdad rtooag): d^ ^4 tsdasa 2 Ood 3 asdodad dza rtao^j
sjAoSo
1. djBdeo eroded dsSo^oi/soaoart e^eabsron
ero^ds ?530dd.dd?.
dsfo.
kod -Sertuad ddodrt^ art
2. saod d37t£)cd wdjsertj-dtfrg
s^do^daeid dsaa&rs^.
3. 'siOdod 12 adoirW aodds4 wc& dsaS&aodo dd/te ddse^ edort adOs».
4. dddo^d ejez^rtd de£> arodeDdsd -d»^ dsaSo^oid^ d^So^zpad
de1?.
5. esde dsak^oid^ eroded dsSotfc&aodrtja d>^^ z^ad ros&bd dxiae^ asazsazb
de^eo 3?2j.
6. dsrt Sedo zorad
&aedoiO
d&oid?,
ra
co Se?uad dx3
_s
4
4 See.
7. esdaod dja3 d.a^oii wdoo ^gesaSo^. esdd erodd ddodaAdd d,do^. uodi
des? doodad dsaSo^ ^?dddd dodjado ^rodsad^an 3s?&.
Q
-C
&.
8. d?rt tsddo ddo droSo^ dead dodd ddr^a rodejadfsa erodedd rort
Q
&
9. e>rtd^dd
az^rtd
A
dsrt R>c3.
d^od
esosfrteb:
? ~’
< A'
► .sea 12 ddoirtVds, a-SI de?dea.
► djad rbodrt <side ddoi 3?ddesadd aa^dodo* do^d zjs?&.
► d?eodaran sadad dds? erocroddrgrt^da, zas?&.
► £>dc& ^?S)d dodd cadd^ doSe^cd zsaodiodde daasaxb zadsddjae
aoda doaeO^.
\
.
.' ..-i;.S«g»... .-A^SgM
■r
IJ
Page 1 of 10
HMJ 2013:346:13263 doi: 10.1136/bmj.13263 (Published 13 June 2013)
RESEARCH
Severe adverse maternal outcomes among low risk
women with planned home versus hospital births in
the Netherlands: nationwide cohort study
OPEN ACCESS
Ank de Jonge midwife senior researcher', Jeanette A J M Mesman physician assistant midwife ,
Judith Mannien senior researcher', Joost J Zwart obstetrician^, Jeroen van Dillen obstetrician , Jos
van Roosmalen professor""
'Department of Midwifery Science, AVAG and the EMGO Institute ol Health and Care Research, VU University Medical Center, Amsterdam.
Netherlands: department of Obstetrics. Leiden University Medical Center. Leiden. Netherlands: deventer Hospital. Deventer. Netherlands:
’’Radboud University Nijmegen Medical Center. Nijmegen, Netherlands; department of Medical Humanities, EMGO, VU University Medical Center,
Netherlands
Abstract
Objectives To les: the hypothesis that low risk women al the onset of
labour with planned home birth nave a higher rate of severe acute
maternal morbidity than women with planned hospital birth, and to
compare the rate of postpartum haemorrhage and manual removal of
placenta.
Design Cohort study using a linked dataset.
Setting Information on all cases of severe acute maternal morbidity in
the Netherlands collected by the national study into ethnic determinants
of maternal morbidity in the netherlands (LEMMoN study). 1 August
2004 to I August 2006. merged with data from the Netherlands perinatal
register of ail births occurring during the same period.
0.63 and 58.3%, 33.2% to 87.5%), the rate of postpartum haemorrhage
was 19.6 versus 37.6 (0.50 0.46 to 0.55 and 47.9%. 41.2% to 54.7%).
and the rate of manual removal ol placenta was 8.5 versus 19.6 (0.41,
0.36 to 0.47 and 56.9%, 47.9% to 66.3%).
Conclusions Low risk women in primary care at the onset of labour
with planned home birth had lower rates of severe acute maternal
morbidity, postpartum haemorrhage, and manual removal ol placenta
than those with planned hospital birth. For parous women these
differences were statistically significant. Absolute risks were small in
both groups There was no evidence that planned home birth among •
low risk women leads to an increased risk of severe adverse maternal
outcomes in a maternity care system with well trained midwives and a
good referral and transportation system.
Participants 146 752 low risk women in primary care at the onset of
labour.
Main outcome measures Severe acute maternal morbidity (admission
to an intensive care unit, eclampsia, blood transfusion of four or more
packed cells, and other serious events), postpartum haemorrhage, and
manual removal of placenta.
Results Overall. 92 333 (62.9%) women had a planned home birth and
54 419 (37.1 %) a planned hospital birth The rate of severe acute
maternal morbidity among planned primary care births was 2.0 per 1000
births.! or nulliparous women the rate for planned home versus planned
hospital birth was 2.3 versus 3.1 per 1000 births (adjusted odds ratio
0.77 95% confidence interval 0.56 Io 1.06), relative risk reduction 25.7%
(95% confidence interval -0.1% to 53.5%). the rate of postpartum
haemorrhage was 43.1 versus 43.3 (0 92, 0.85 to 1 00 and 0.5%, -6.8%
to 7.9%). and the rate of manual removal of placenta was 29.0 versus
29 8 (0.91.0.83 to 1.00 and 2.8%. -6.1% to 11.8%). For parous women
the rale of severe acute maternal morbidity for planned home versus
planned hospital birth was 1.0 versus 2.3 per 1000 births (0.43, 0.29 to
introduction
Thu relative safety of planned home births is a topic of
continuous debate.1 Several studies have compared severe
adverse perinatal outcomes among planned home births with
those of planned hospital births.' 5 The rate of adverse perinatal
outcomes was low and not significantly dilterent in most
studies.'1’ although slightly higher for primiparous women with
planned home births in a recent large cohort study ' The authors,
however, disagreed about the interpretation of these results.2 6 7
Less evidence is available on the association between planned
place of birth and maternal morbidity, especially severe adverse
maternal outcomes, since these are rarer than severe adverse
perinatal outcomes. Several studies have shown that at the onset
of labour low risk women with planned home births have lower
rates of referral to secondary care, augmentation, medical pain
relief, operative delivery, postpartum haemorrhage, and
episiotomy than women with planned hospital births. ''3 ' •
Correspondence to: A de Jonge ank.de|onge@vumc.nl
No commercial reuse See rights and reprints
Subscribe-
Page 2 of 10
8MJ2013;3'l6:f3263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
RESEARCH
_ ____ ___
Some have questioned the rationale of routine hospital birth for
low risk women because of the exposure to overuse of medical
interventions with potentially harmful effects.1 However,
although the overall rate of maternal complications may be
lower among planned home births, the delay due to
transportation from home to hospital might lead to severe acute
maternal morbidity. A previous Dutch study showed that the
lower rate of medical interventions is not an impoilant reason
for women to choose a home birth, but sense of safety is a
dominant reason to choose a hospital birth."' Therefore, even
though the rate of severe acute maternal morbidity is small, if
the risk would be higher among planned home births this would
probably be a reason for many women to choose a hospital birth.
As far as we know, no studies have been large enough to study
severe acute maternal morbidity among planned home births.
Of all Western countries, the Netherlands has the highest
percentage of home births and is therefore ideally suited to study
the association between planned place of birth and rare but
severe outcomes.’ " National obstetric, midwifery, and neonatal
data are recorded in the Netherlands perinatal register. In
addition, the national study into ethnic determinants of maternal
morbidity in the netherlands (the LEMMoN study; Landelijke
studie naar Etnischc verschillen in Maternale Morbiditeit in
Nederland) resulted in a database of all cases of severe acute
maternal morbidity in the country over two years.13 Merging
data from the national perinatal register and LEMMoN databases
provided us with a unique opportunity to compare the rate of
severe acute maternal morbidity among planned home births
and planned hospital births. In addition, we compared the rale
of postpartum haemorrhage and manual removal of placenta.
The main hypothesis was that low risk women in primary care
al the onset of labour with planned home birth have higher rates
of severe acute maternal morbidity than those with planned
hospital birth.
Methods
In the Netherlands, midwives in primary care provide care to
low risk women. These are women with a singleton pregnancy
of a fetus in cephalic presentation who do not have any medical
or obstetric risk factors that are an indication for secondary care,
such as previous caesarean section, and who start labour
spontaneously between 37 and 42 weeks.
If complications or risk factors occur during pregnancy, labour,
or after birth, women are referred io secondary care. After
referral, women may receive care from clinical midwives,
obstetricians, obstetric registrars, and obstetric nurses, under
the final responsibility of an obstetrician. Obstetric interventions
such as electronic fetal monitoring, augmentation, and medical
pain relief only take place in secondary care. The indications
for referral are laid out in the obstetric indication list.1- This list
is revised regularly by a project group consisting of midwives,
obstetricians, paediatricians, and general practitioners.
Women who are still in primary care al term can choose to give
birth at home or in hospital, assisted by their primary care
midwife. Women with a “medium risk" indication can give
birth in primary care but are advised to give birth in hospital.
The official medium risk indications according to the obstetric
indication list are postpartum haemorrhage or retained placenta
after a previous birth.1; Midwives may record other reasons for
medium risk if they think it is better for a woman to give birth
in hospital.
No commercial reuse: See rights and reprints
Data linkage
We combined the information from the datasets of the LEMMoN
study and the national perinatal register. The methods of the
LEMMoN study have been described in detail elsewhere.12 In
short, all cases of severe acute maternal morbidity were collected
from all 98 hospitals in the Netherlands over two years (1
August 2004 to 1 August 2006). Each month a local coordinator
reported all cases, or the fact that there were no cases, via a web
based form.
The national perinatal register database consists of data from
three separate databases: one for primary care (national perinatal
database-1), one for secondary care (national perinatal
database-2), and one for paediatric care (national neonatal
register). During 2004-06 an estimated 95-99% of primary
midwifery care practices and 99-100% of hospital based
obstetric practices entered data into the perinatal register.111'’
The three datasets are combined into one national perinatal
database via a validated linkage method.1' We selected all data
from the national perinatal register for the period in which the
LEMMoN study took place.
In both databases we selected women with a singleton pregnancy
without a history of caesarean section who gave birth between
37 and 42 weeks and had spontaneous onset of labour. We only
included cases in the LEMMoN study if severe acute maternal
morbidity occurred after the onset of labour.
Primary linkage of data from both datasets was based on date
of birth of the baby plus or minus two days and date of birth of
the woman. If there was more than one match or if date of birth
of the baby was missing in one of the datasets, we used the
following additional variables for matching: postpartum
haemorrhage more than 1000 mL. hospital number, and postal
code. Two researchers (AJ and J Ma) checked whether the data
were well matched. We compared the characteristics of
LEMMoN cases that were not linked with the national perinatal
register with those that were linked.
We excluded women who were referred during labour from
primary io secondary care but were missing the form from
primary care, owing to important information, for example on
their planned place of birth, being unavailable. We compared
the characteristics of these women with the total sample to
examine differences between the two groups.
Study sample
For the analyses we selected women who were in primary care
at the onset of labour. We excluded women who were referred
because of ruptured membranes for more than 24 hours without
contractions since their planned place of birth did not have an
effect on their labour process. To ensure that groups were as
comparable as possible, we excluded all women with a record
of a “medium risk" indication.
The study sample therefore consisted of women in primary care
with a term singleton pregnancy without a medium risk
indication, prolonged ruptured membranes without contractions,
or any indication for secondary care at the onset of labour.
Definition of variables
The variable for planned place of birth comprised three
categories: planned home birth, planned hospital birth, and
unknown planned place of birth. At some point during pregnancy
the midwives in primary care register women’s planned place
of birth in the national perinatal database-1. This information
is missing for some women: midwives may forget to record the
Subscribe: ■:
Page 3 of 10
BMJ 2013:346:13263 doi 10.1136/bmj.l3263 (Published 13 June 2013)
RESEARCH
details or the women may not have made a decision on where
to give birth until the onset of labour.
The main outcome variable was severe acute maternal morbidity,
which was defined in the LEMMoN study in five different
categories: admission to intensive care, uterine rupture,
eclampsia or HELLP (haemolysis, elevated liver enzymes, and
low platelet count) with liver haematoma, major obstetric
haemorrhage (blood transfusion of four or more packed cells),
and other severe acute maternal morbidity as diagnosed by the
attending clinician. Secondary outcomes were the individual
categories ol severe acute maternal morbidity; we combined
uterine rupture and other indications in the category
“miscellaneous.” Other secondary outcomes were postpartum
haemorrhage more than I()()() mL and manual removal of
placenta, both based on data from the perinatal register.
We identified the following confounders that may be associated
with planned place of birth and with maternal complications:
parity, gestational age, maternal age. ethnicity, and
socioeconomic position.1" IK19 Parity was coded as nulliparous
or parous. Gestational age was divided into 37 to 37+6 weeks,
38 to 40+6 weeks, and 41+0 to 41 +6 weeks. Maternal age was
coded as less than 25. between 25 and 34, and 35 or older. The
ethnicity classification is challenging in the perinatal
register- for example, women of Turkish or Moroccan
background are both classified as “Mediterranean” and women
of African origin are classified by some midwives as “creole”
and by others as "other.'' We therefore categorised ethnicity as
Dutch and non-Dutch. Socioeconomic position was derived
from social status scores based on postal codes developed by
the National Institute for Social Research based on income,
employment, and level of education. These scores were divided
into low (below 25th centile). medium (between 25th and 75th
centile), and high (above 75th centile).
Augmentation of labour with oxytocin and operative delivery
(caesarean section, vacuum, or forceps delivery) have been
associated with adverse maternal outcomes.1"ISIn a secondary
analysis we therefore controlled the results for augmentation of
labour and operative delivery (vacuum, forceps, or caesarean
for primary and secondary care, but this information is not
always consistent. We conducted sensitivity analyses for women
without discrepancies between data from primary and secondary
care for this variable and for onset of labour based on the
national perinatal database-1 only.
Results
Linkage of data
During the study period, 240 400 women who had no previous
caesarean section, a singleton pregnancy, and a spontaneous
onset of labour between 37 and 42 weeks' gestation were
recorded in the national perinatal register. In the LEMMoN
study, 706 women met these criteria and had severe acute
maternal morbidity after the onset of labour (27.7% of all
women with severe acute maternal morbidity) (figure j). Of
these, 56 could not be linked to data in the perinatal register
(7.9%<). Women with severe acute maternal morbidity who were
linked to the perinatal register did not differ significantly for
type of severe acute maternal morbidity, parity, and ethnicity
from those that were not linked to the register.
Of the total linked data. 10 101 (4.2%) women were referred
during or after labour but were missing the national perinatal
database-1 form and 52 of the women in this category had severe
acute maternal morbidity. Compared with all women who were
referred during or after labour these women were more likely
to be parous (31.29<- r 30.0%) and of Dutch ethnicity (83.4% v
78.7%). There were no significant differences between these
groups in incidence and type of severe acute maternal morbidity.
The linked dataset contained information on 230 299 women,
of whom 598 (2.6 per 1000) had severe acute maternal
morbidity. Of these. 172 973 started labour in primary care
(severe acute maternal morbidity, n=364), and for 439 women
(severe acute maternal morbidity, n-1) the level of care at the
start of labour was unknown.
Study population
We used SAS version 9.2 to merge data, and analysed the data
using SPSS version 19.0. Within eaeh planned place of birth
category we calculated the number and percentage of the primary
Of the women in primary care at the onset of labour, planned
place of birth was unknown for 18 070 and these women were
not included in the analyses (fig I). Another 2112 women were
excluded because they had a “medium risk" indication. Of these,
1248 (59.1 %) had a history of retained placenta or postpartum
haemorrhage and the others had various indications such as “no
prenatal care” and “use of medication (not further specified).”
and secondary outcomes. We performed logistic regression
analyses only for severe acute maternal morbidity, blood
An additional 6039 women were not included because they were
referred for prolonged ruptured membranes without contractions.
transfusion of four or more packed cells, postpartum
haemorrhage, and manual removal of placenta, because of a
low number of events in the other outcomes; these analyses
were done for nulliparous and parous women separately and lor
planned home births versus planned hospital births. For all of
Of the remaining 146 752 women in primary care al the onset
of labour. 92 333 (62.9%) had a planned home birth and 54 419
(37.1%') had a planned hospital birth (table 1 ). Women with
planned home birth compared with those with planned hospital
section).
Data analyses
these outcomes we present the crude odds ratios anil 95%
confidence intervals. We used multivariable logistic regression
analyses to control for potential confounders. resulting in
adjusted odds ratios with 95% confidence intervals. We also
present relative risk reductions with 95% confidence intervals.
Subsequently, the associations between planned place of birth
and severe acute maternal morbidity were controlled for
augmentation of labour with oxytocin and operative delivery
(both as binary variables). We excluded missing data because
they were less than 5(X for all variables.
For the main analyses we used the perinatal register definition
of onset of labour in primary or secondary care. Onset of labour
is defined in the register based on information from the databases
No commercial reuse See rights and reprints
birth were more likely to be parous, less likely to give birth
between 37+0 and 37+6 weeks' gestation, and more likely to
give birth between 41+0 and 41+6 weeks; they were less often
younger than 25 years, more often aged between 25 and 34
years, more often of Dutch origin, and less often of a lower
socioeconomic position.
Adverse maternal outcomes
Of all women included in the analyses, 288 (2.0 per 1000) had
severe acute maternal morbidity (table 2i ). Among planned
home births, severe acute maternal morbidity occurred in 141
women (1.5 per 1000) and among planned hospital births in
147 women (2.7 per 1000). Most of the affected women had a
blood transfusion of four or more packed cells. Other causes
Subscribe:
a ■ i.-subscfiticj
Page 4 of 10
BMJ2013:346.f3263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
RESEARCH
were rare. Postpartum haemorrhage was the most common
adverse maternal outcome and this occurred among 2699 (29.2
per I ()()()) planned home births and among 2172 (39.9 per 1000)
planned hospital births.
Adverse outcomes were less common among planned home
births than among planned hospital births, but differences were
only statistically significant for parous women (table 3 J.
Among nulliparous women outcomes for planned home versus
planned hospital births were: severe acute maternal morbidity
adjusted odds ratio 0.77 (95% confidence interval 0.56 to 1.06)
and relative risk reduction 25.7% (95% confidence interval
-0.1 % to 5.3.5%), blood transfusion of four or more packed
cells 0.90 (0.65 to 1.27) and 14.5%' (-14.7% to 45.8%),
postpartum haemorrhage 0.92 (0.85 to 1.00) and 0.5% (-6.8%
to 7.9% ). and manual removal of placenta 0.91 (0.83 to 1.00)
and 2.8% (-6.1%- to 1 1.8% ). Among parous women outcomes
for planned home versus hospital births were: severe acute
maternal morbidity adjusted odds ratio 0.43 (95% confidence
interval 0.29 to 0.63), blood transfusion of four or more packed
cells 0.45 (0.30 to 0.68), postpartum haemorrhage 0.50 (0.46
to 0.55). and manual removal of placenta 0.41 (0.36 to 0.47).
Sensitivity analyses and adjustment for
medical interventions
Sensitivity analyses showed similar results for all outcomes in
table 3 (data not shown). In some of the sensitivity analyses,
differences just reached statistical significance that did not in
the main analyses. For example, for the comparison of severe
acute maternal morbidity, if only women without discrepancies
in onset of labour between the data forms from primary and
secondary care were selected the adjusted odds ratio for planned
home versus planned hospital birth in nulliparous women was
0.63 (95% confidence interval 0.44 to 0.88) and in parous
women was 0.46 (0.30 to 0.69). If onset of labour was based on
the national perinatal database-1 form only, the differences in
severe acute maternal morbidity, postpartum haemorrhage, and
manual removal of placenta became significant for nulliparous
women: 0.72 (0.53 to 0.99). 0.90 (0.83 to 0.97). and 0.88 (0.80
to 0.96). respectively.
Fewer women with planned home births compared with planned
hospital births received augmentation of labour (nulliparous
women 22.9%- r 27.5% and parous women 3.4%. v 7.8%;.
respectively) and had an operative delivery (nulliparous women
23.1%. i’ 24.7% and parous women 1.6% v 3.2%. ). The
comparison of severe acute maternal morbidity controlled for
augmentation of labour and operative delivery for planned home
versus planned hospital births among nulliparous women gave
an adjusted odds ratio of 0.80 (0.58 to 1. 10), which is an increase
of 3.9%. in odds ratio. For parous women the adjusted odds ratio
for severe acute maternal morbidity after controlling for these
interventions was 0.47 (0.32 to 0.69). which is an increase of
9.3% in odds ratio.
Discussion
Low risk women in primary care at the onset of labour who
planned to give birth al home had lower rales of severe acute
maternal morbidity, postpartum haemorrhage, and manual
removal of placenta compared with women who planned to give
birth in hospital, but the differences were only statistically
significant for parous women. Odds ratios for severe acute
maternal morbidity changed slightly when we adjusted the
results for medical interventions, and more so for parous than
for nulliparous women.
No commercial reuse. See rights and reprints
Strengths and limitations of this study
A major strength of our study is the large sample size and the
fact that all cases of severe acute maternal morbidity that
occurred in all hospitals in the Netherlands were collected
meticulously over two years. As far as we are aware, this is the
largest study to date into the association between planned place
of birth and severe adverse maternal outcomes.
Our study has some limitations as well. Firstly, because we used
registration data, some were missing or may have been
misclassified. For example, information on the variable “start
of labour in primary or secondary care” was not always
consistent between midwifery and obstetric registration.
However, sensitivity analyses using different definitions of this
variable generated similar results. In addition, 10 101 women
were excluded because their national perinatal database-1 form
was missing when they were referred during labour. Some of
these women were cared for by general practitioners or midwives
who do not participate in the national perinatal registration. In
particular, general practitioners who still practise midwifery are
often located in rural areas. This may explain the higher rate of
parous women and women of Dutch ethnicity among those with
a missing national perinatal database-1 form. For 18 070 women
planned place of birth al the onset of labour was unknown. Their
rate of severe acute maternal morbidity was comparable to that
of women who planned hospital births. Even if all of these
women would have a planned home birth or. alternatively, if
all of them would have a planned hospital birth, the strength of
the associations would have changed but the results would have
been in the same direction.
Secondly we collected the data from 2004 to 2006 and
theoretically midwifery management and women’s
characteristics may have changed. However, we have no reason
to believe that at present planned home birth leads to more
unfavourable maternal outcomes. For example, the percentage
of women with a singleton pregnancy who were older than 35
years only increased from 20.5%; in 2004 to 21.1% in 2006 and
this percentage was 21 A% in 2010." 1 Besides, we controlled
the results for differences in maternal age.
Thirdly, although none of the women who started labour in
primary care should have had an indication for secondary care
according to the obstetric indication list, there may still have
been differences in risk profiles between women who planned
labour at home versus in hospital. We corrected the analyses
for known risk factors, such as maternal age and ethnicity.
Adjusting the results regarding severe acute maternal morbidity
for augmentation of labour and operative delivery only led to a
small reduction in the differences. This means that medical
interventions explain some of the differences in severe acute
maternal morbidity, which is consistent with earlier studies that
showed higher rates of adverse maternal outcomes among
women with medical interventions.1’ I!' 11 However, the fact that
odds ratios for adverse maternal outcomes were much lower for
parous women than for nulliparous women, suggests that other
factors played an important part. Those women who had a
relatively difficult previous birth may have been more likely to
plan a hospital birth next lime, even if there was no official
medical indication. If so, this self selection may have resulted
in better outcomes among women with planned home birth. In
addition, there may have been residual confounding owing to
differences in characteristics that could not be identified. For.
example, we had no information on body mass index. Although
a high body mass index is not an official medium risk indication
according to the obstetric indication list, midwives may have
advised these women to give birth in hospital. They may have
ticked the medium risk box but they could not record body mass
Subscribe:'^
Page 5 of 10
BMJ 2013:346:13263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
RESEARCH
1
index as the reason for medium risk in the national perinatal
database-1.11
Nevertheless, our hypothesis that low risk women at the onset
of labour who planned birth at home would have a higher rate
of severe acute maternal morbidity compared with women who
planned birth in hospital was not confirmed. Women with
planned home birth had lower rates of all adverse maternal
outcomes, albeit not significantly so for nulliparous women.
This is consistent with other studies that found lower rates of
maternal morbidity among planned home births.’4 ’22 Concern
about safety is an important reason for women to choose hospital
birth, and even more so for their partners."’ '' They worry
especially about transportation to hospital in case of an
emergency. However, although the referral rate during labour
is high in the Netherlands, only 3A(7< of women are referred for
urgent reasons.’* Our results suggest that planned home birth
for low risk women is not associated with an increased risk of
adverse maternal outcomes despite the possible delay in case
of an emergency. Previous studies have not shown higher risks
of severe adverse perinatal outcomes either for planned home
births compared with planned hospital births in the
Netherlands.'" We should emphasise that our results may only
apply to regions where midwives are well trained to assist
women al home births and where facilities for transfer of care
and transportation in case of emergencies are adequate. In 2009,
82% of women were in hospital within 45 minutes from the
moment a midwife called an ambulance in an emergency
situation.’5 The average time was 35 minutes (standard deviation
12 minutes). Travelling lime Io hospital is important for the
.safely of all births, regardless of planned place of birth. A Dutch
study showed that the incidence of adverse perinatal outcomes
was higher if travel time from home to hospital was more than
20 minutes, but differences were only statistically significant
for women in secondary care at the onset of labour.'''
Planned hospital births are also associated with risks. The rate
of medical interventions is lower for planned home versus
planned hospital births among low risk women: for example,
odds ratios for caesarean section varied between 0.31 and 0.76
in different studies. 1 '' It is important to limit the use of
caesarean section because of its association with various adverse
outcomes at the current birth, and the risk of uterine scar rupture
during the next pregnancy and birth.12 2,12'29 However, again
selection bias may play a part despite all women in these studies
being considered al “low risk.” Although more women with
planned hospital birth may have needed interventions to ensure
a good perinatal outcome, considering the large size of the
differences in the rate of medical interventions between the
groups, it is unlikely that these can be explained by a difference
in risk profile only.
The fact that we did not find higher rates of severe acute
maternal morbidity among planned home births should not lead
to complacency. Every avoidable adverse maternal outcome is
one too many. An audit of maternal morbidity should be used
to learn from every case of severe acute maternal morbidity to
improve care, optimise the risk selection system, and prevent
future severe acute maternal morbidity from happening.’"
We thank the Netherlands perinatal registry for the use of the national
database.
Contributors: AJ conceived the study, wrote the article, and is guarantor
of the study. JMe and AJ conducted the analyses. JMa linked the
datasets. All authors contributed to interpretation of the data, critically
revised earlier drafts of the paper for important intellectual content, and
gave final approval of the version to be published. The researchers had
access to all the research data.
Funding- This study was funded with a career grant (VENI) from ZonMw.
The funder had no role in any aspect of the study.
Competing interests: All authors have completed the ICMJE uniform
disclosure form at www.icmje.org/coi._disclosure.pdf (available on
request from the corresponding author) and declare: no support from
any organisation for the submitted work; no financial relationships with
any organisations that might have an interest in the submitted work in
the previous three years: no other relationships or activities that could
appear to have influenced the submitted work.
Ethical approval: The ethical committee of VU University Medical Center
confirmed that ethical approval was not necessary for this study
(reference No 11/399).
Data sharing: No additional data available.
2
3
4
5
Our study showed a lower risk of severe acute maternal
morbidity, postpartum haemorrhage, and manual removal of
placenta among low risk women in primary care at the onset of
labour with planned home versus planned hospital births. These
differences were statistically significant for parous women. We
found no evidence that planned home birth among low risk
.
Olsen O. Clausen JA. Planned hospital birth versus planned home birth. Cochrane
Database Syst Kev2012;(9) CD000352.
Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned
place ot birth for healthy women with low risk pregnancies: the Birthplace in England
national prospective cohort study. BMJ 2011:343:d7400.
De Jonge A, Van der Goes BY. Ravelh AC. Amelink-Verburg MP. Mol BW. Nijhuis JG, et
al. Perinatal mortality and morbidity in a nationwide cohort ot 529,688 low-risk planned
home and hospital births. BJOG 2009:116:1177-84.
Hutton EK. Reitsma AH. Kaufman K. Outcomes associated with planned home and
planned hospital births in low-risk women attended by midwives in Ontario. Canada.
2003-2006: a retrospective cohort study. Birth 2009;36:180-9.
Janssen PA Saxell L. Page LA. Klein MC. Liston RM. Lee SK. Outcomes ol planned
home birth with registered midwile versus planned hospital birth with midwife or physician.
6
CMAJ 2009.181:377-83.
Van der Kooy J. Poeran J. De Graaf JP. Birnie E. Denktass S. Steegers EA, et al. Planned
home compared with planned hospital births in the Netherlands intrapartum and early
7
neonatal death in low-risk pregnancies. Obsrel Gynecol2011;118:1037-46.
Olferhaus P. Rijnders M, De Jonge A. De Miranda E. Planned home compared with
planned hospital births in the Netherlands: intrapartum and early neonatal death in low-risk
8
9
10
11
12
13
14
15
16
Conclusion
No commercial reuse: See rights and reprints
women leads to an increased risk of severe adverse maternal
outcomes in a maternity care system with well trained midwives
and a good referral and transportation system.
17
18
19
pregnancies. Obstel Gynecol2012; 119(2 Pt 1):387-8.
Davis D. Baddock S. Pairman S. Hunter M, Benn C. Wilson D. et al. Planned place of
birth in New Zealand: does it affect mode ol birth and intervention rates among low-risk
women? Birth 2011 ;38:111-9.
Lindgren HE. Radestad IJ, Christensson K. Hildingsson IM. Outcome of planned home
births compared to hospital births in Sweden between 1992 and 2004. A population-based
register study. Acta Obstet Gynecol Stand 2008:87:751-9.
Van Haaien-Ten Haken T. Hendrix M, Nieuwenhuijze M, Bude L. De Vries R. Nijhuis J.
Preferred place of birth: characteristics and motives of low-risk nulliparous women in the
Netherlands. Midwilery 2012:28:609-18.
Christiaens W. Nieuwenhuijze MJ De Vries R. Trends in the medicalisation of childbirth
in Flanders and the Netherlands. Midwilery 2013;29:e1-8.
Zwart JJ. Richters JM. Ory F. De Vries JI, Bloemenkamp KW. van Roosmalen J. Severe
maternal morbidity during pregnancy, delivery and puerpenum in the Netherlands: a
nationwide population-based study ol 371.000 pregnancies BJOG 2008:115:842-50.
Obstetric Vademecum. Final report of the Maternity Care Committee of the College of
health insurance companies. [Verloskundig Vademecum. Eindrapport van de Commissie
Verloskunde van het College voor zorgverzekeringenj. De Koninklijke Nederlandse
Orgamsatie van Verloskundigen (KNOV). De Landehjke Huisartsen Verenigmg (LHV).
De Nederlandse Veremging voor Obstetne en Gynaecologie (NVOG). Zorgverzekeraars
Nederland (ZN), Inspectie voor de Gezondheidszorg, eds. 2003. Diemen.
Slichtmg Perinatale Registratie Nederland. Perinatal Care in the Netherlands 2004
[Perinatale zcrg in Nederland 2004], 2007. Stichling Perinatale Registratie Nederland.
Slichting Perinatale Registratie Nedeiland. Perinatal Care in the Netherlands 2005
[Perinatale Zorg m Nederland 2005]. 2008. Slichting Perinatale Registratie Nederland.
Slicntmg Perinatale Registratie Nederland. Perinatal Care m the Netherlands 2006
[Perinatale zorg in Nederland 2006]. 2008. Slichting Perinatale Registratie Nederland.
Meray N. Reitsma JB. Ravelli AC, Bonsel GJ, Probabilistic record linkage is a valid and
transparent tool to combine databases without a patient identification number. J Clin
Epidemiol 2007:60 883-91.
Kramer MS Dahhou M, Vallerand D, Liston R, Joseph KS. Risk factors for postpartum
hemorrhage: can we explain the recent temporal increase’’ J Obstet Gynaecol Can
2011:33:810-9.
Zwart JJ, Jonkers MD, Richters A, Ory F. Bloemenkamp KW. Duvekot JJ. et al. Ethnic
disparity in severe acute maternal morbidity: a nationwide cohort study in the Netherlands.
Eur J Public Health 2011:21:229-34
Subscribe:
Page 6 of 10
BMJ 2013:346:13263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
. .
RESEARCH
-i
What is already known on this topic
Low risk women with planned home birth at the onset of labour have lower rates of referral from primary to secondary care during labour,
augmentation, medical pain relief, operative delivery, postpartum haemorrhage, and episiotomy than those with planned hospital birth
Studies so far have been too small to compare severe acute maternal morbidity between planned home birth and planned hospital birth
among low risk women
What this study adds
Low risk women in primary care with planned home birth at the onset of labour had a lower rate of severe acute maternal morbidity,
postpartum haemorrhage, and manual removal of placenta than those with planned hospital birth
These differences were statistically significant for parous women
There was no evidence that planned home birth among low risk women leads to an increased risk of severe adverse maternal outcomes
in a maternity care system with well trained midwives and a good referral and transportation system
20
21
22
23
Van Dillen J. Zwart JJ. Schutte J. Bloemenkamp KW. Van Roosmalen J. Severe acute
maternal morbidity and mode of delivery in the Netherlands. Ada Obstel Gynecol Scand
2010,89.1460-5.
Stichtmg Perinatale Registralie Nederland. Perinatal Care in the Netherlands 2010
|Perinatale Zorg in Nederland 2010|. 2013. Stichting Perinatale Registrat
Blix E. Huitfeldl AS, Oian P, Straume B, Kumle M. Outcomes of planned home births and
planned hospital births in low-risk women in Norway between 1990 and 2007: a
retrospective cohort study. Sex Reprod Healthc 2012:3:147-53.
Bedwell C. Houghion G. Richens Y, Lavender T. She can choose, as long as I m happy
with it: a qualitative study ol expectant fathers views of birth place. Sex Reprod Healthc
24
2011.2'71-75.
Amelink-Verburg MP. Verloove-Vanhorick SP, Hakkenberg RM. Veldhuijzen IM.
Bennebroek GJ. Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices,
25
a descriptive study. BJOG 2008:115:570-8.
Kommer GJ, Zwakhals SLN. Time in ambulance care. Analysis of emergency journeys
in 2009. (Tijdsduren in ambulancezorg. Analyse van spoedinzelten in 2009.]. RIVM, ed.
26
RIVM Briefrapport 270482001/2010. 2010.
Ravelli AC. Jager KJ, De Groot MH. Erwich JJ. Rijninks-van Driel GC, Tromp M. et al.
Travel lime from home to hospital and adverse perinatal outcomes in women al term in
Declercq E. Barger M. Cabral HJ. Evans SR. Kotelchuck M, Simon C. et al. Maternal
outcomes associated with planned primary cesarean births compared with planned vaginal
28
births. Obstel Gynecol2007:109:669-77.
Declercq E. Cunningham DK, Johnson C. Sakala C. Mothers’ reports of postpartum pain
associated with vaginal and cesarean deliveries: results of a national survey. Birth
29
2008:35:16-24.
Fitzpatrick KE. Kurinczuk JJ. Alfirevic Z. Spark P. Brocklehurst P. Knight M. Uterine rupture
by intended mode ol delivery in the UK: a national case-control study. PLoS Med
30
2012;9:e1001184.
Van Dillen J. Mesman JAJM. Zwart JJ. Bloemenkamp KW, Van Roosmalen J. Introducing
maternal morbidity audit in the Netherlands. BJOG 2010:117:416-21.
Ciie this as: WJ2013;346:f3263
This is an Open Access article distributed in accordance with the Creative Commons
Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute,
remix, adapt, build upon this work non-commercially. and license their derivative works
on different terms, provided the original work is properly cited and the use is
non-commercial. See: http://creativecommons.Org/licenses/by-nc/3.0/.
the Netherlands. BJOG 2011 ;118:457-65.
No commercial reuse: See rights and reprints :
27
■. ■ r
Subscribe:
•■/ ■■/v/.-; c-ni.orwovz w. !
BMJ 2013;346:f3263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
Page 7 of 10
RESEARCH
Tables
| Characteristics of low risk women in primary care at onset of labour
Characteristics
Total (n=146 752) Planned place of birth at onset of labour
Home (n=92 333)
Hospital (n=54 419)
Parity:
0
65 227 (44.4)
38 728 (41.9)
26 499 (48.7)
It
81 521 (55.6)
53 602 (58.1)
27 919(51.3)
4(0)
Missing data
Gestational age:
5700 (3.9)
3404 (3.7)
2296 (4.2)
38+0 to 40+6
107 763 (73.4)
67 507 (73.1)
40 256 (74.0)
41+0 10 41+6
33 289 (22.7)
21 422 (23.2)
11 867 (21.8)
37+0 to 37+6
Maternal age (years):
<25
18 549 (12.6)
9142 (9.9)
9407(17.3)
25-34
101 691 (69.3)
66 554 (72.1)
35 137 (64.6)
>35
26 498 (18.1)
16 630(18.0)
9868 (18.1)
14(0)
Missing data
Ethnicity:
Dutch
119 755 (82.0)
83 629 (90.9)
36 126 (66.9)
Non-Dutch
26 289 (18.1)
8385 (9.1)
17 904 (33.1)
Missing data
708 (0.5)
Socioeconomic position:
High
35 567 (24.6)
23 243 (25.5)
12 324 (23.0)
Medium
66 419 (45.9)
45 320 (49.7)
21 099 (39.4)
Low
42 861 (29.6)
22 671 (24.8)
20 190 (37.7)
Missing data
1905 (1.3)
For all characteristics P<0.001.
| No commercial reuse: S6e rights and reprints http.■Avww.i.ytij eom/permissiOfis
Subscribe: htip-Vwwwbmi.convstJbscritHn
Page 8 of 10
BMJ 2013:346:13263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
RESEARCH
•_
| Severe acute maternal morbidity, postpartum haemorrhage, and manual removal of placenta in low risk births starting in primary
care: total group
No with outcome (No/1000 women)
Outcomes
Total (0=146 752)
Planned home birth (n=92 333) Planned hospital birth (n=54 419)
Severe acute maternal morbidity
288 (2.0)
141 (1.5)
147(2.7)
Admission to intensive care unit
70 (0.5)
32 (0.3)
38 (0.7)
19 (0.1)
8(0.1)
11 (0.2)
Eclampsia or severe HELLP syndrome
Blood transfusion >4 packed cells
256(1.7)
134 (1.5)
122 (2.2)
Postpartum haemorrhage (>1000 mL)
4871 (33.2)
2699 (29.2)
2172 (39.9)
2865 (19.5)
1550(16.8)
1315 (24.2)
Manual removal ol placenta
HELLP=haemolysis, elevated liver enzymes, and low platelet count.
Missing data: postpartum haemorrhage 1234 (0.8%), manual removal ot placenta 2106 (1.4%).
Women could have more than one type of severe acute maternal morbidity.
No commercial reuse See rights and reprints
Subscribe:
Page 9 of 10
BM/2013;346:f3?.63 doi: 10.1136/bmj.13263 (Published 13 June 2013)
RESEARCH
| Severe acute maternal morbidity, postpartum haemorrhage, and manual removal of placenta among low risk nulliparous and
parous women starting labour in primary care
Nulliparous women (n=65 227)
Variables
Parous women (n=81 521)
Planned home birth (n=53 602)
Planned hospital birth
(n=27 919)
Planned home birth (n=38 728
Planned hospital birth
(n=26 499)
89 (2.3)
82 (3.1)
52(1.0)
65 (2.3)
Reference
Severe acute maternal morbidity:
No (No/1000)
Crude odds ratio (95% Cl)
0.74 (0.55 to 1.00)
Reference
0.42 (0.29 to 0.60)
Adjusted odds ratio (95% Cl)
0.77 (0.56 to 1.06)
Reference
0.43 (0.29 to 0.63)
Reference
25.7 (-0.1 to 53.5)
Reference
58.3 (33.2 to 87.5)
Reference
85 (2.2)
68 (2.6)
49 (0.9)
54 (1.9)
Reference
Relative risk reduction (%. 95% Cl)
Blood transfusion >4 packed cells:
No (No/1000)
Crude odds ratio (95% Cl)
0.86 (0.62 to 1.18)
Reference
0.47 (0.32 to 0.70)
Adjusted odds ratio (95% Cl)
0.90 (0.65 to 1.27)
Reference
0.45 (0.30 to 0.68)
Reference
Relative risk reduction (%. 95% Cl)
14.5 ( 14.7 to 45.8)
Reference
52.7 (24.9 to 85.3)
Reference
Postpartum haemorrhage:
1655 (43.1)
1134 (43.3)
1044 (19.6)
1038 (37.6)
Crude odds ratio (95% Cl)
1.0 (0.92 to 1.07)
Reference
0.51 (0.47 to 0.56)
Reference
Adjusted odds ratio (95% Cl)
0.92 (0.85 to 1.00)
Reference
0.50 (0.46 to 0.55)
Reference
Relative risk reduction (%. 95% Cl)
0.5 (-6.8 to 7.9)
Reference
47.9 (41.2 to 54.7)
Reference
1099 (29.0)
773 (29.8)
451 (8.5)
542(19.6)
Crude odds ratio (95% Cl)
0.97 (0.89 to 1.07)
Reference
0.43 (0.38 to 0.48)
Reference
Adjusted odds ratio (95% Cl)
0.91 (0.83 to 1.00)
Reference
0.41 (0.36 to 0.47)
Reference
Relative risk reduction (%. 95% Cl)
2.8 (-6.1 to 11.8)
Reference
56.9 (47.9 to 66.3)
Reference
No (No/1000)
Manual removal of placenta:
No (No/1000)
Adjusted relative risks adjusted for variables in table 1.
No commercial reuse: See rights and reprints
Subscribe:
• fx I
Page 10 of 10
I3MJ 2013,346:13263 doi: 10.1136/bmj.f3263 (Published 13 June 2013)
RESEARCH
Figure
National perinatal register data
Singleton pregnancies, spontaneous onset of labour
from 37 to 42 weeks' gestation without
history of caesarean section (n-240 400)
LEMMoN data
Singleton pregnancies, spontaneous onset of labour, and severe
acute maternal morbidity (SAMM) after onset of labour
from 37 to 42 weeks' gestation without history of
caesarean section (n>=706; 27.7% of total LEMMoN cases)
b
LEMMoN cases not linked to
perinatal register data (n=56; 7.9%)
I....
Linked data total (n-240 400), SAMM (n-650; 2.7/1000)
Primary care form missing for women referred during labour (n”10 101; 4.2%), SAMM (n“52; 5.1/10OO)
Eligible women (n»23O 299), SAMM (n-598; 2.6/1000)
Primary care at onset of labour
(n=172 973), SAMM (n-364; 2.1/1000)
Secondary care at onset of labour
(n=56 887), SAMM (n=233; 4.1/1000)
Unknown level of care at start of labour
(n=439), SAMM (n«l; 2.3/1000)
Excluded (n-26 221; 15.2%), SAMM (n-76; 2.9/1000):
Planned place of birth unknown (n»18 070), SAMM (n»=46; 2.5/1000)
Medium risk at onset of labour (n»2112), SAMM (n-10; 4.7/1000)
Prolonged ruptured membranes, no contractions (n=6039). SAMM (n=20; 3.3/1000)
Total for comparison within primary care (n-146 752), SAMM (n-288; 2,0/1000)
---------------------------J--------------------------- }
Planned home at onset of labour (n-92 333; 62.9%),
SAMM(n«141; 1.5/1000)
Planned low risk hospital at onset of labour (n-54 419; 37.1%),
SAMM (n”147; 2.7/1000)
Flow of births between August 2004 and July 2006
No commercial reuse See righls and reprints
p
Subscribe:
‘'
Policy Recommendations for Maternal Health in India
The Delhi statement
Context of maternal health and maternal mortality in India
Maternal mortality continues to be an unjustifiably significant problem in India in spite of the issue garnering a
lot of attention and being the focus of policy and programme by the Government of India and international
bodies. Health activists have been feeling increasingly dissatisfied with the maternal health care situation on the
ground in India. Many women continue to die around child birth because health facilities in many parts of the
country are not equipped to provide Emergency Obstetric Care, the quality of antenatal care provided is
inadequate, and safe abortion services in the public sector are inaccessible for the majority of women.
Government reports, however, project that the maternal health situation is improving mainly because the Janani
Suraksha Yojana disbursements are increasing.
We believe that women have the right to the highest attainable standards of maternal health and
maternal health care. Maternal health services have to be available, accessible, acceptable, and of good
quality. While motherhood is often a positive and fulfilling experience, for too many women it is associated with
suffering, ill-health and even death.
The approach to addressing maternal health in India is fragmented and focused on promoting institutional
deliveries alone, while overlooking the broader framework of sexual and reproductive rights. The maternal
health policy in India needs to move away from the paradigm of institutional deliveries to a paradigm of safe
deliveries. Several issues that affect maternal health - such as access to safe abortion services, access to choice of
contraception, dignified childbirth, poverty, nutrition remain blind spots in policy.
Similarly, gender based violence is a crucial factor that has major health implications and even death. This is the
result of physical injuries and also of barriers created by domestic violence to women seeking appropriate care
during pregnancy and delivery. The situation is exacerbated by the state’s regressive demographic goals and
coercive population policies that have dictated health policies and programmes for women especially in terms of
financingand resource allocation. There is enough evidence to suggest that attention to ante natal and postnatal
care has suffered because ofthe priority accorded to the family planning programme in the country.
Thus, the solutions proposed often fail to capture or be relevant to the lived realities of women. Approaches to
reduction of maternal mortality have for too long been driven by experts, funders and international bilateral
organizations, with the voices of the women of India and the activists working among them, hardly ever being
included in policy and programme planning. Maternal mortality reduction strategies have been target oriented
and treat maternal mortality as a simple input - output problem. In the pastyear or so, there have been a number
of documentations of maternal deaths by civil society groups from different parts of India including from the so
called ’developed' states like Tamilnadu, Karnataka and Kerala. All of these reports bring out the inadequacy of
purely technical and narrow indicator-oriented approaches, without concurrent attention to the social
determinants, health systems and other broader aspects surrounding these deaths.
While Maternal Death Reviews are mandated and are being done in several states, many maternal deaths still fail
to get reported, especially those that occur outside hospital settings. There is no public disclosure of the analysis
of maternal deaths, or of the measures planned to address the causes of maternal deaths. Neither is there an
1
■ ■
accurate and disaggregated database from which the especially vulnerable groups can be identified.
In order to focus more political attention to maternal health in the country and to suggest recommendations for
policy and programmes, a group of public health specialists and civil society activists from different networks
and organizations including CommonHealth, NAMHHRand Jan Swasthya Abhiyan, SAMA, CEHAT, SOCHARA and
SAHA) met in Delhi on the 12th and 13th August, 2013. The meeting saw the participation of nearly forty persons
working closely at the grassroots on issues related to maternal health. On the second day, Dr Syeda Hameed,
Member, Planning Commission and Mr Keshav Desiraju, Secretary, MoHFW, interacted with civil society
members at a policy dialogue session. The sessions saw several concerns and recommendations emerging from
the consultation.
Ourconcerns
•
In spite of the fact that the poorest and most vulnerable women are the most affected, the government
has fallen short in addressing maternal health with a comprehensive strategy and being accountable for
it.
•
Maternal Death Reviews, though mandated since 2010, have not been institutionalised in many districts
across various states, and are notbeing carried out in several communities, especially in rural areas.
•
Even where maternal death reporting and reviews are being done, this information is not available in the
public domain so as to ensure transparency and accountability of the process.
• Important social determinants like poverty, caste and gender including violence against women that
have been shown repeatedly by civil society documentations to be intimately related to maternal health
and maternal mortality, are not being addressed in any manner by existing programmes.
• There is a lack of institutionalized systems of accountability to the community in the health system
including for critical issues like maternal mortality.
• Undignified treatment of women, especially those from marginalized communities, during childbirth
has been reported from various parts of the country, but is not acknowledged as a problem. Women
report facing physical abuse and verbal abuse, particularly use of derogatory, sexually explicit language.
This makes them reluctantto use public health facilities thus impacting access.
• Gender based violence, including domestic violence, which is known to have an impact
impact on
on women's
women s
control over their fertility, as well as pre and post partum health of mothers is not even addressed as an
issue of concern.
• Unsafe abortion which is a major cause of maternal mortality is not adequately addressed in maternal
health programmes.
Key Recommendations
/
In spite of the increase in the number of institutional deliveries in recent years, quality of care remains a
serious concern. Marginalized women from vulnerable caste groups and geographically remote areas
continue to be excluded from programmes. Therefore, we recommend that
• Ensuring SAFETY must be the priority in ALL deliveries irrespective of where they occur and who
conducts them.
2
11
I
•
Outcome indicators should go beyond JSY disbursements and number of institutional deliveries to
include indicators of Safety such as, completeness of antenatal care, technical aspects of care like
Active Management of Third Stage of Labour and provision of postpartum care.
•
Blood availability continues to be an important issue. Blood storage units should be operationalized at
every FRU.
• Referrals are often done unnecessarily and to facilities that do not have the capacity to manage specific
complications. Availability of emergency transport during such referrals is also an important issue.
Accountability during referrals must be ensured and continuity of care provided during transit between
facilities during referrals. Ensuring that women are accompanied by appropriately trained health
personnel during such referrals, providing free emergency transport, and instituting audits of referral
protocols and outcomes are some mechanisms to ensure accountability duringreferrals.
• Verbal and physical abuse by health care providers, during labour in public health facilities must be
stopped and action taken against health care providers who indulge in it. Mechanisms to address
grievances particularly related to abuse must be put in place in health systems.
/
Policies and programmes must respond to women's needs that go beyond quality health care during
pregnancy, delivery and post partum period to include nutrition, contraception, access to safe abortion,
freedom from violence, dignity during care and access to information and care, from adolescence
throughouttheir life span.
•
Documentations of maternal deaths show that non-obstetric causes are becoming an important
contributor to maternal deaths. Services for tuberculosis, malaria and rheumatic heart disease
during pregnancy must be strengthened and integrated with existing vertical programmes for
these diseases.
• Availability and access to abortion services in the public health sector need to be ensured.
Information on number of abortion services provided in public sector facilities should be collected and
analysed.
/
Policies and programmes need to be more nuanced and tailored to the needs of women in different
situations.
•
For instance, screening for sickle cell anaemia in tribal populations, bed nets and malaria
prophylaxis in malaria endemic areas.
•
Maternal health care needs to be placed in the context of all-round strengthening of health systems.
•
Maternal health care can be strengthened only within a functioning primary health care system and
Universal Access to Health Care that is publicly provisioned and tax-financed.
• While the Janani Shishu Suraksha Karyakram is a step towards Universal Maternity Care, this should
be monitored rigorously both from within the system and through communities to ensure that no out
ofpocket expenditures are being incurred.
/
Maternal health is dependant on a range of social determinants like nutrition, gender, poverty, caste,
religion.
3
I
•
Needs of pregnant women should be prioritized in all social welfare programmes at all levels. (For
example, adding maternity benefits in NREGA)
• Specific interventions like one fresh cooked meal women in pregnancy and during lactation as
demonstrated in Andhra Pradesh should be implemented.
• Screening of gender based violence during pregnancy should become an integral part of antenatal
care.
/
The state has to be accountable for ensuring the health of every woman during pregnancy and delivery
including access to safe abortion services if necessary.
• Ensure social audits including provision of resources and setting up mechanisms.
• Quality of care in the private sector needs to be monitored and regulated.
•
Make Maternal Death Reviews transparent and accountable. Strengthen reporting systems for
maternal deaths by including reporting from persons outside the health system like Anganwadi
workers, teachers, PRI members and self help group members.
—
Broaden district and state MDR committees to include civil society representatives, PRIs and
independenttechnical experts.
-
Include private sector deaths in MDR
Consolidated reports of MDRs should be made public with details of actions recommended and
taken.
Tools should be modified to include better evidence for technical details and also social
determinants.
• Ensure grievance redress mechanisms, including immediate response systems and district level
ombudspersons.
This statement is an outcome ofdiscussions during the National Consultation on Maternal Health in India,
held in Delhi on August 12 and 13,2013 organized by the undersigned organizations.
CommonHealth
Coalition for Maternal-Neonatal Health and Safe Abortion
www.commonhealth.in
Alt
People's Health Movement
JSA
Sama
NAMHHR
Date of Publication July 2014
4
building community health
Iv>
(
01 032 ch26.qxd 05/24/2005 18:05 Page 1
*
Chapter 26
Maternal and Perinatal Conditions
Wendy J. Graham, John Cairns, Sohinee Bhattacharya. Colin H W
Bullough. Zahidul Quayyum. and Khama Rogo
The Millennium Declaration includes two goals directly rele
vant to maternal and perinatal conditions: reducing child mor
tality and improving maternal health. The fact that two out of
the eight Millennium Development Goals (MDGs) are exclu
sively targeted at mothers and children is testament to the sig
nificant proportion of the global burden of disease they suffer
and to the huge inequities within and between countries in the
magnitude of their burden. Achieving these goals is inextrica
bly linked at the biological, intervention, and service delivery
levels (Bale and others 2003).
*
Maternal and child health services have long been seen as
inseparable partners, although.over the past 20 years the rela
tiveemphasis within each, particularly at a policy level, has var
ied (De Brouwere and Van Lerberghe 2001). The launch of the
Safe Motherhood Initiative in the late 1980s, for example,
brought heightened attention to maternal mortality, whereas
the Internauona! Conference Qn Population and Development'
CPD) broadened the focus to reproductive health and, more
recently, to reproductive rights (Germain 2000). Those shifts
can be linked with international programmatic responses and
erminology—with the preventive emphasis of, for instance,
prenatal care being lowered as a priority relative to the treat
ment focus of emergency obstetric care. For the child, inte
tanagement of
of childhood
childhood illnesses
has bro.wht
grated management
”
renewed eimphasis to maintaining a balance between preventive
and curative
ive care. The particular needs of the newborn how
ever, have only started
past three or four years (Foege 2001).
Although health experts agree that the single clinical inter
vent,ons needed to avert much of the burden of maternal and
perinatal death and disability are known, they also accept that
these interventions require a functioning health system to have
an effect at the population level. Levels of maternal and perina
tal mortality are thus regarded as sensitive indicators of the
entire health system (Goodburn and Campbell 2001), and they
can therefore be used to monitor progress in health gains more
generally. What is also clear is that maternal mortality and the
neonatal component of child mortality continue to represent
two of the most serious challenges to the attainment of the
MDGs, particularly in South Asia and Sub-Saharan Africa.
An estimated 210 million women become pregnant each
year, and close to 60 million of these pregnancies end with the
death of the mother (500,000) or the baby or as abortions.
I his chapter focuses on the adverse events of pregnancy and
childbirth and on the intervention strategies to eliminate and
ameliorate this burden.
EPIDEMIOLOGY OF MATERNAL
AND PERINATAL CONDITIONS
Much has been written about the lack of reliable data on
and perinatal conditions in developing countries
,
J2003’('rah;'m 200TSave the Children 2001). Weak
rdi- "'e ‘"f0"™'1™ ’yStems’ inadeq“a<e vital registration, and
non, I r-0"
10USCl,°ld SUrveys as ,he main
of
Recognizing the implications of these obstacles for prioritizing
health needs and interventions is important and is now
endorsed by a global movement toward evidence-based deci
sion making for policy and practice (Evansand Stansfield 2003)
I
I
'..••P 001 -032 ch26.qxd 05/24/2005
18:05
Page 2
However, there has been much less appreciation of the conse
quences for evaluations of effectiveness—arid thus cost
effectiveness—of the weaknesses in current outcomes mcasure^ntandinmutinedatacollecticin.Thiiseweakncssesalsoaffect
the monitoring of progress toward the MDGs. Initiatives for
improved health surveillance arc thus urgently needed (CMH
2002). lor the vast majority of the worlds population, the magmtuc c o adverse maternal and perinatal outcomes is not known
reliably. It ,s impossible to determine whether many of the pat
terns apparently observed, especially at a cause-specific level
arc real or are artifacts of the measurement process
Definitions
The terms maternal and perinatal encompass a continuum of
health states-from the most positive (complete physical, men
tal, and social well-being) to the most negative-and a huge
number of clinical conditions. This chapter focuses on eight
major condmons, hereafter referred to as the focus conditions.
v nch are estimated to account for about 75 percent of mater
nal deaths and more than 60 percent of perinatal deaths. For
he mother, these condmons are hemorrhage, sepsis, hyperten
sive disorders of pregnancy, obstructed labor, and unsafe abor'o-i. For the baby, they are low birthweight, birth asphyxia, and
infection (table 26.1).
We define maternal conditions as encompassing events
1992a?nTSb
b"1 Conception t0 42
Postpartum (WHO
b , k P7S °n WOmen’S hea“h' familT Planni»g. ado
lescent health, and surgery address the longer-term sequelae of
perinatal period can happen at any age, although it tends to
take place during the neonatal period (up to 28 days of life). By
contrast, perinatal deaths include both stillborn babies and
those who are born alive but die before the end of the seventh
day. Early neonatal deaths only include live births.
Nature and Characteristics
Pregnancy and childbirth are not inherently pathological.
Maintaining an effective balance, however, between preserving
normality and ensuring a state of readiness to deal with abnor
mality represents a fundamental challenge to health systems
and a tension in safe motherhood programming. Although this
balance between prevention and treatment is not peculiar to
maternal and perinatal conditions (or complications), the fol
lowing additional characteristics are relevant to assessing the
burden as well as the effectiveness of interventions:
* The principle of “first, do no harm” has particular signifi
cance in this area, because many preventive practices related
tO pr^nancy and childbirth can readily become harmful in
unskilled hands—for example, inappropriately early induc
tion of labor or poor forceps technique. The iatrogenic bur
den of maternal and perinatal conditions is rarely factored
into assessments of intervention effectiveness.
The lives of two individuals, mother and baby, are poten
tially at stake (Stoll and Measham 2001); however, interven
tions will not necessarily benefit both equally, and indeed,
some will be in direct conflict.
’ A ‘T ,Tber of matcrnal “d Perinatal conditions present chmcally not as smgle entities but as complexes, such as
hemorrhage and sepsis or preterm delivery and birth
asphyxia For the mother, the situation may be further com-
pregnancy and childbirth; the p-- _r
preconception period; pregnan9’ at an early age; and specific interventions, such
—, —.1 as repair of *
obstetric fistulas. Within the period from c...„
conception to 42 days
postpartum, two broad categories of condition
*-^ns can be distinguished: those arising specifically from pregrjnancy and parturibon {direct obstetric conditions), and those
Em ".I0 ,r°le °f Underlying conditions’ sud> “
ose aggravated by \
H1V/AIDS underlying puerperal sepsis.
or aggravating to pregnancy (indirect obstetric <
- • '
-—...c
conditions).
Because the latter conditions, such as malaria,
HIV/AIDS
or ' • The most extreme negative outcome, death of both the
t mother and the baby, is highly
anemia, are not exclusive to pregnant or parturient women
c ,
•
° -7 concentrated around the
they a. not deaft with here but in the relevant disease-specific
tune of del,very, from the onset of labor or abortion to
48 hours postpartum or postabortion. Estimates indicate
hat about two-thirds of maternal deaths occur within this
Regarding perinatal conditions, we focus on those for which
tervenbons can be directed to the baby through the mother
by "he disc8nanCy
VCry- °Ur diSCUSSi°n is “"’Pkmented
b)
discussion in chapter 27, which concentrates on the
oeonate, mcludmg special care of the small baby and emer
gency care of the sick newborn.
Formal definitions of perinatal conditions tend to vary by
d«a source. Taken literally, they refer to conditions that arise in
perinatal period (Murray and Lopez 1998), which are not
he same as events that occur in the perinatal period-Xt is
from 28 days of gestation to the end of the seventh day of life’
■«r example, death resulting from conditions that arise in the
t.mc window (AbouZahr 1998), and the proportion for
perinata! deaths appears to be even higher (Bale and others
les 'brrt 7”°
’h0WeVer’3 gr°Wil’8 number °f
>es h.ghhght the contribution of direct and indirect causes
of deaths, mcludmg violence, when a one-year postpartum
and'othXf MOU
KOdi°’
19"; H°'
• The initial clinical presentation of some conditions can be
thZe W,th.ri,p‘d7aiation 10 a life-threatening state, and
.
CQndltlons often require surgical intervention
• A d.stmct ehnieal feature of some maternal conditions is
then unpredictability (AbouZahr 1998). This fact has had a
2 • Wendy J. Graham. John Cairns. Sohinee Bhatlacharya. et al.
I
o
i”0
o
2
|NJ
Table 26.1 Maternal and Perinatal Focus Conditions and Risk Factors for These Conditions
Condition
Case
fatality rate*
(percent)
Average duration
until death if
condition fatal
Antepartum hemorrhage,
bleeding from the genital tract
during the last 3 months of
pregnancy
Not
available
12 hours
Primary postpartum hemorrhage;
excessive bleeding (more than
500 milliliters) from the genital
tract following delivery
1.0
Definition or complications
and sequelae
n
w
<n
_______Risk factors for of condition
Distal or Direct
proximate
physiological
Timing of
presentation
Risk factors for death from condition
Distal or
Direct
proximate
physiological
Maternal
Hemorrhage
2 hours
28 weeks of gestation
up to delivery
Delivery to 24 hours
after delivery
Primigravidity
/
Fibroids
Placental abnormalities
(including placenta previa;
abruption; placenta
accreta, percreta. increta;
other adhesions)
Anemia
Polyhydramnios
Grand multiparity
(greater than 4)
Remote location
Anemia
Coagulopathies
x
Q.
2
£o
Lack of blood
transfusion
in
Badly managed third
stage of labor
S
2
Delay or absence of
oxytocic treatment
*0
0>
10
<T>
Multiple gestation
Previous third-stage
complication
Previous cesarean section
Preeclampsia, eclampsia
Intrauterine death
Hepatitis
Induced labor
Prolonged labor
Precipitate labor
Forceps delivery
Cesarean section
1
Chorioamnionitis
Disseminated intravascular
coagulation
Sepsis
r
a
I
i
co
Infection of the genital tract or
extragenital infections folio-wing
childbirth
1.3
6 days
Delivery to 6 weeks
postpartum
Immunosuppression
Prolonged labor
Anemia
Obstructed labor
Sexually transmitted
infections
Premature rupture
rupture of
of
Premature
membranes
Immunosuppression antibiotics
Inadequate prenatal
care
Frequent pelvic
examinations
Lack of knowledge intravenous
about
signs ant^'Otics
about warning
wai
huaute.™ death
Lack of postnatal
care
,
Delivery by
Misdiagnosis
untrained personnel inappropriate use of
Anemia
■
Foreign body insertion
(for example, herbs)
’ lack of access to
~
-
■
Cultural practices
Instrumental delivery
(Continues on the following page.)
)
s
g
Table 26.1 Continued
I
lI
2
fj
Condition
Definition or complications
Case
fatality rate*
and sequelae
(percent)
Average duration
until death if
Timing of
condition fatal
presentation
________Risk factors for of condition
Distal or
Direct
Risk factors for death from condition
Distal or
Direct,
proximate
proximate
f
£L
Unhygienic delivery
J
S
conditions
o
Retained products of
in
conception
Hypertensive
disorders of
Raised blood pressure with
1.7
proteinuria
2 days (eclampsia)
i
28 weeks of gestation
Extremes of maternal
to 2 days postpartum
age
Primigravidity
Genetic predisposition
pregnancy-induced
care
Racial or geographical
hypertension or chronic
hypertension
pregnancy
2
predisposition
Multiple gestations
Cultural practices
Appearance of
Molar pregnancy
Lack of knowledge
Previous history of
Lack of prenatal
complications, such as
cardiovascular and
cerebral complications,
Labor in which progress is----------- 0.7
-3 days
’
During labor-----------
Disseminated
intravascular
Lack of prenatal care
coagulation
arrested by mechanical factors
Cephalopelvic-------- —
Rickets in childhood
disproportion
. Lack of access to
cesarean deLvery
Bony deformity of
Malpresentation. position
Lack of access to
Hemorrhage
instrumental
delivery and
Sepsis
pelvis
Achondroplasia
symphysiotomy
Short stature
Uterine rupture
Exhaustion,
dehydration
Scarred uterus
Primigravidity
Inappropriate use
Grand multiparity
of oxytocin
Adolescent pregnancy
Unsafe abortion
Procedure for terminating an
0.3
6 hours to 6 days
After first missed
Unwanted pregnancy
Absence of aseptic
Sociocultursi
Perforated uterus
Adolescence
technique
factors
out by people lacking the
period to 22 weeks of
gestation or fetal
Poisoning from
necessa.7 skills or in an
environment that dees not
weight of less than
unintended pregnancy earned
conform to minimal medical
standards or both
500 grams
Unmarried
Foreign body insertion
Lack of access to
abortifacients
Absence of legal
Poisoning from
safe terminason
Peritonitis
abortion services
abortifacients
services
Lack of access to
contraception
Lack of access to safe
abortion services
Sexually transmitted
infections
Q>
IQ
a>
platelets syndrome
Diabetes and chronic
hypertension
Malnutrition----------
CD
s
hemolysis, elevated
liver enzyme, low
Eclampsia
Obstructed labor
<T>
physiological
Cesarean section
f
f
physiological
Lack of access to
postabortion ^re
Septic shock
Acute renal failure
Hepatorenal failure
3owel injury,
perforation
Hemorrhagic shock
Peritonitis
I
S
Perinatal0
n
Low birthweight (less Respiratory insufficiency in
than 2.500 grams)6
preterm infants with lung
immaturity presenting as
respiratory distress syndrome
because of surfactant deficiency
50
Neonatal cerebral injury caused
by periventricular hemorrhage
mediated by perinatal stress
such as hypotension or trauma
80
Severe physiological jaundice of
preterm infant
50
5 days
3 days
1-5 days
Less than 24 hours
1-4 days
2-5 days
J
Difficulties in establishing
spontaneous feeding and
inability to tolerate feeds
resulting from prematurity
20
First day
3 days to months
3-14 days
Race, ethnicity
Short interpregnancy
interval
Low socioeconomic
status
First or second trimester
bleeding
Lack of knowledge
and understanding
Unmarried
Placenta previa
Respiratory infection
Respiratory distress
syndrome
Multiple pregnancy
Lack of education
Preeclampsia
Parity (0 or greater
than 4)
Anemia
Smoking, alcohol
Isoimmunization
Maternal malnutrition
Fetal abnormalities
Genetic factors
__ i
Birth
Central nervous system
injury
$
$
in
Cervical incompetence
i
=
I
I
20 minutes
Birth (5 minutes)
Neonatal encephalopathy:
clinically evident disturbance in
neurological behavior, commonly
with early neonatal seizures in
term babies, resulting from an
event causing hypoxia during
delivery
30
3 days
Birth-first 12 hours
Maternal diabetes
*0
a>
ua
n>
in
Neonatal coagulopathy
I
Poor obstetric history
Drugs taken during
labor, including
anesthesia
CD
s
Sudden infant death
syndrome
r
High altitude
20
o
Other infections
Oligohydramnios or
polyhydramnios
Diethylstilbbestrbl.
other toxic exposure
Absent or depressed breathing
at birth
s
Cholestatic liver
disease
Absent or inadequate
prenatal care
Birth asphyxia
(excluding birth
trauma)
a
Intraventricular
hemorrhage
Necrotizing
enterocolitis
Hyperemesis
Rubella, other viral
infection
_____________Hypoglycemia and other
_ 2
metabolic disorders related to
prematurity _
Birth asphyxia
Lack of adequate
neonatal care
facility
Maternal diabetes or
hypertension
failure of closure of the ductus
70
arteriosus, frequently seen in
preterm babies with lung disease
I
1-14 days
s
Extremes of maternal
age
Prolonged or obstructed
labor
Badly conducted
labor
Central nervous system
injury
Abruptio placentae
Lack of fetal
monitoring
Neonatal
encephalopathy
(seizures and recurrent
apnea)
Maternal hypertension
Placental infarct.
insufficiency
Preeclampsia
Postmaturity
Any other severe
illness
Prematurity or low
birthweight
Lack of partograph
Lack of neonatal
resuscitation
facilities
Multiple pregnancies
Placenta previa or
separation
Cord prolapse
(Continues on the following page.)
cn
Table 26.1 Continued
f
f
i
I
f
I
=L
L
Condition
Infection
Case
Average duration
fatality rate* until death if
Timing of
(percent)
- condition fatal
presentation
2______ Risk factors for of condition
Distal or
Direct
proximate
physiological
Risk factors for death from condition
Distal or
Direct,
proximate
physiological
Neonatal sepsis of early onset
resulting from intrauterine or
intrapartum infection
30-40
Lack of adequate
prenatal care
Premature rupture of
membranes
Congenital HIV
infection
Neonatal sepsis of late onset
resulting from nosocomial
infection er lack of immunity to
commensal bacteria
15
Maternal infection
Preterm delivery
Lack of maternal
immunization
Birth asphyxia
Lack of adequate
neonatal care
Tetanus neonatorum, commonly
resulting from unhygienic cutting
of the cord or care of the cord
stump
80
3-7 days
3-14 days
Congenital syphilis resulting
from transolacental infection
with Treponema pallidum after
18 weeks gestation
30
5 days
Birth onward
Definition or complications
and sequelae
HA/ infection transmitted either
intrapartum or postpartum
5 days
5 days
First 3 days
After 3 days
Unhygienic cultural
practices
Unhygienic delivery and
cord care
Preterm delivery
5
Septic shock
Respiratory failure
Hepatorenal failure
1
Coagulopathies
Direct effects mainly
after neonatal penod
Cha'noerla,n
■995; Case t3:a,i,yrates:
i—:•
.OWU11U- u^u^u .door w ’994; UnS3'5 530,1Cn: WH0 1992b; ,ow ‘’^weight: Bate and others 2003. Roberta 19S3. Yasmin ana
_______________
’■*
orers 2001, birth asphyxia: Bale and
otners 2003 p. 324. Robertson 1993; mfect.ons. Rooertson 1993; risk factors: Calder and Dunlop 1992. Murray and Lopez 1998.
b SaMstillbinns00,nteRS‘',e'S8'ai,aCl8’beCaUSe'h'S‘StherOrrnSo“rhAs'aar°Afnc3c. Includes preterm deliveries and small for gestational age.
1
I
I
<1 -O?;? ch26.qxd
05/24/2005
18:05
Page 7
*
profound effect on the prioritization of interventions in safe
motherhood, and it is an area in urgent need of further
research. 1 he situation is confused by the alternative end
points, such as death or disability, and by the extent to which
there are clear and predictable risk factors. Table 26.1 sum
marizes some of these key characteristics as they relate to the
eight focus conditions.
developing country (1 in 6) and the lowest estimate for a devel
oped country (1 in 29,800) (WHO 2004b). This differential is
often cited as the largest discrepancy between the developed
and developing world of all public health statistics, reflecting
major differences both in obstetric risk, as measured by the
maternal mortality ratio, and in levels of fertility, as reflected in
the total fertility rate.
In terms of medical causes of maternal mortality, even
greater caution is needed regarding the reliability of any pat
terns observed, because of their dependence on whether the
data are health service based or population based and on cod
Causes and Conceptual Frameworks
One of the most frequently quoted figures in safe motherhood
is that 88 to 98 percent of maternal deaths are avoidable with
moderate levels of health care (WHO 1986). This advocacy
statement simplifies the multiple pathways leading to death
and, thus, the multiple opportunities for primary and second
ary prevention. In part, this simplicity is a further reflection of
the grouping together of clinical conditions that in reality are
distinctly different in terms of prevalence, case fatality, and
scope for intervention, such as eclampsia and puerperal sepsis
or congenital anomalies and birth asphyxia. The multiple end
points and conditions, for both the mother and the fetus or
newborn, have implications for what is regarded as an
antecedent (a cause, a determinant, or a risk factor)1 and what
is regarded as a consequence (an outcome or a sequela).
A large number of conceptual frameworks depict pathways
to adverse maternal and perinatal outcomes (Bale and others
2003; McCarthy and Maine 1992). Several identify three levels
of contributory factors, which are also found in causal models
for general health outcomes (WHO 2002): (a) distal, (b) prox
imal or intermediate. and (c) physiological or direct. Table 26.1
highlights the risk factors for the focus maternal and perinatal
conditions. The distal determinants emphasize that maternal
and perinatal well-being is not . just a medical issue
Improvements throughout the health sector must be comple
mented by attention to wider social, economic, and cultural
actors as well as to reproductive rights (CMH 2002). Many
conceptual frameworks also differentiate between the timing of
interventions: before pregnancy, during pregnancy, during
labor and delivery, or during the postpartum period. Similarly,
a further distinction can be made in terms of the timing of the
outcome, although from
i a programmatic perspective, such a
temporal focus may lead to fragmented care for women and
their babies.
ing conventions. Figure 26.1a shows the percentage distribu
tion among direct causes at a crude global level. Direct causes
account for about 80 percent of all maternal deaths, with indi
rect causes responsible for the remainder. Of the direct causes,
hemorrhage is generally regarded as the most common and
may be underestimated, because health facilities are unaware of
many such deaths, given the short interval between onset and
death (sec table 26.1). In terms of indirect causes, the pattern
varies enormously between different parts of the world, prima
rily according to the prevalence of HIV/AIDS, malaria, and
tuberculosis.
The published data on severe maternal morbidity arc
weaker still. A recent World Health Organization (WHO) sys
tematic review indicates how prevalence figures vary hugely
accordmg to the criteria used to identify cases (Say, Pattinson,
and Gulmezoglu 2004). Using disease-specific criteria, WHO
found that prevalence ranged from 0.80 to 8.23 percent Using
organ syste.n criteria, WHO found that the range was 0.38 to
1.09 percent. Finally, using management-based criteria, WHO
found that the range was 0.01 to 2.99 percent. Estimates sug
gest that for every maternal death, at least 16 or 17 other
women suffer a life-threatening complication during preg
nancy or childbirth (Gay and others 2003) and at least 30
women are left with long-term disabilities, such as an obstet
ric fistula (UNFPA 2003). These estimates must be regarded as
crude approximations, most originating from small-scale
studies and most m urgent need of updating and verification
Gwen the varying case fatality
fatality rates
rates shown
shown in
in table
table 26.1,
26 I, the
fact 'hat the distributional
pattern for
for morbidity (fig
........I pattern
ure 26. b) does not completely
completely mirror
mirror the
the one for mortality is
not surprising.
z
As concerns mortality in babies, an estimated 5.7 million
permatal deaths occur each year, 47 percent as stillbirths ami
53 percent m the first week of life (J. Zupan, personal commumcation, August 25, 2004). Many of those deaths are linked
d.rect y w.th complications experienced by the mothers, and
several studies have shown that the survival prospects for a
baby whose mother dies are generally poor-less than 1 per
>
Levels, Trends, and Differentials
The iatat regional estimates for maternal mortality are for
■2(100 (table 26.2), with most of the figures for the developing
world produced by modeling (WHO 2004b). More than 99 per
cent of annual maternal deaths occur in the developing world
A a ttattonal level, the magnitude of the differential in Lns of
I'fetnne nsk is almost 500-fold between the highest figure for a
cent m one Study in Bangladesh (Koenig, Fauveau, and
OJ ymak 991). In 2004, neonatal deaths represented 36 per
cent of all deaths of children under five in developing
Maternal and Pminatal Conditions I 7
I
’ r'P 001 -032 Ch26.qxd 05/24/2005
18:05
Page 8
Table 26.2 Estimates of Maternal Mortality by Region, 2000
Number of
maternal deaths
as modeled
by WHO
Number of
maternal
deaths, 2001
Central and Eastern
Europe. Commonwealth
of Independent States.
Baltic states. Europe,
and Central Asia
Lifetime risk
of maternal
death (1 in
number shown)
64
3.400
3,000
770
29
100
1.6
East Asia and the
Pacific
110
37,000
360
44
210
2.0
Eastern and
Southern Africa
980
15
490
1.500
5.5
Latin America and
the Caribbean
190
16,000
160
110
280
2.6
Middle East and
North Africa
220
15.000
100
85
380
South Asia
3.7
560
940
900
199,000
237.000
43
370
400
760
16
1,500
16
310
1,600
3.5
5.7
5.9
8
230
17
1.6
680
3.0
Region
Sub-Saharan Africa
Western and
Central Africa
37,000
123.000
22.000
21.000
205.000
240.000
118.000
Total
fertility
rato
High-income countries
13
Low- and middle
income countries
1,300
1.000
440
4,000
527.000
507,000
61
Low-income countries
890
World
236.000
400
17
529.000
410
508.000
1,400
5.4
74
210
620
2.7
Source. WHO ZOOM. UN 2002; WHO 2004b.
— = not available.
to. The w a,B taa uarf by ihB
r
Natas
neonatal
births remain particularly poor.
.re^sdanT” °f inf<“ndearth of reliable
ends data ,s hard y surprising. At a global level, a major diffi
culty nses from the need to use models to estimate maternal
over tim ' 11 .baSiC 'nethodol°8y'he models has changed
tver time, the data are not appropriate for trend assessment
AbouZahr and Wardlaw (2001) provide patchy support for
ownward trends m some parts of the world, mostly on the
b. ms of civil registration data and mostly restricted to countries
bir s™ h" Th li,y T1105 °f'eSS ,han 100 P" “KWO live
births-thus notably excluding South Asia and Sub-Saharan
fnca. Even where declines appear to have occurred, they did so
poor to 1990 Countries with sustained falls since then su “
Argennna and China, cannot be regarded as represent ive of al
developing countries. Cause-specific trend data are extremel
rare, often gathered through small-scale hospital-based studies
8
Range of uncertainty of
maternal mortality
rate estimates
Lower
Upper
estimate
estimate
Maternal mortality
ratio (maternal
deaths per 100,000
live births)
WHO 2004 1 'eS
'
eXamP'e’ Pa,tinS°n 2OO2)- Rc“">
jWHO (2004c) statistics on unsafe abortion show an apparent
leaffi r C ‘n
in ail WOrld re8io“. though the risk of
death remains htgh at 50 per 100,000 live births, and in parts of
h a rn
b the riSk iS as high as 140 P" 1OO'“° 'ive
births (Rogo, Bohmer, and Ombaka 1999). These adverse
events however are often also the most seriously underreportcd, as elaborated further in chapter 57
The availability of reliable trends data for perinatal mortalX is even more problematic. A demand for population-based
b“Lffi nC1bOrnS iS COmParative,y
‘bus, there has
been msufficient time to accumulate multiple data points
ueniograplnc and health surveys (DHSs) are a key source fi.r
tracking trends in infant and child mortality. Several DHSs
now ave ata that can be disaggregated to show neonatal
ea hs, but only a few have information on stillbirths, and the
from WHO su ;nf°™atiOn St'11
assessed. Information
fr
OQ
ggests that ear,y ne°natal death rates fell slightly
28 per 1,000 live births around 1980 to about 25 per 1,000
■n 2000, for low- and middle-income countries, and the
Wend, J Catan. John Cairns. Sah.nea Bhanacharya. el al.
I
1
'2 Ch26.qxd 05/24/2005
18:05
Page 9
a. Maternal mortality
equivalent trend lor stillbirths is suggested to be a drop from 3b
per I,()()() deliveries to 22 per 1,000 deliveries (J. Zupan, per
sonal communication, August 25, 2004).
Two types of differentials are particularly relevant: geo
graphic (or regional) and socioeconomic. Table 26.2 indicates
the wide variation in the magnitude of maternal mortality
across regions, and a similar difference can be seen between
countries. In terms of absolute numbers of deaths, just 13
countries account for 70 percent of the global total (WHO
2004b).2 Caution is again needed, because the poorest coun
tries also have the weakest information systems and, therefore,
have estimates derived solely from modeling. One regression
model (WHO 2004b), for example, uses independent variables,
such as the percentage of deliveries with health professionals
present and the proportion of deaths of women of reproduc
tive age that are maternal deaths. Those variables are them
selves subject to error and likely to be least reliable where infor
mation systems are weakest. Geographic differences in mater
nal mortality within countries are poorly documented,
although remote populations are often assumed to suffer the
highest levels because of poor access to emergency obstetric
care. Although this assumption seems logical, few reliable data
are available to confirm or refute it, and the possibility of high
levels of mortality in urban areas linked to unsafe abortion
(Thonneau and others 2002) makes the topic of geographic
differentials a priority for research.
Until recently, socioeconomic differentials in mortality have
tended to be inferred from utilization patterns for prenatal care
and health professionals at delivery. The DHSs continue to provtde the main data sources in this regard, for both internation
al and national analyses, and they demonstrate huge differences
between wealth quintiles. A relevant recent development
however, is the familial technique, which can be used to
Other maternal
22%
A
Hemorrhage
<
28%
Unsafe abortion
13%
Obstructed
labor
8%
Sepsis
15%
Hypertensive
disorders
14%
b. Maternal morbidity
"^Hemorrhage
\ 18%
Other maternal
22%
X
Sepsis
16%
Unsafe abortion
26%
Obstructed
labor
9%
Hypertensive
disorders
9%
Wore: Nonobstetric (indirect) causes of death and morbidity, such as tuberculosis
and malaria, have been excluded.
Source: Mortality: WHO 2004d; Morbidity: Murray and Loper 1998.
Figure 26.1 Medical Causes of Direct Maternal Mortality
and
Morbidity (percentage distribution)
examme socioeconomic differences in maternal mortality
using existing survey data (Graham and others 2004). Because
Table 26.3 Early Neonatal Deaths
by Gender and Cause, 2001
(thousands)
I
WorldCause
All
Perinatal conditions6
•
2.523
t
1
South Asia
Sub-Saharan Africa*
Male
Female
All
Male
Female
1.400
AH
Male
1.123
Female
1.086
' 757
597
490
573
332
241
406
351
243
141
102
122
70
240
139
101
68
90
52
38
Low birthweight1
1.301
710
Birth asphyxia
(including birth trauma)
591
739
432
307
Other perinatal conditions'1
482
258
‘
192
225
137
Soi«n> WHO 2004(1
a Excludes the island of Mayotte.
68
c Includes preterm deliveries and^XlHo'r'^taZara'gr3™5' C°n9en"al SVPhiliS' aCqi"fed in,eC"°nS ,resPira'orV and sepsis), and diarrhea.
condmons orrg.nat.ng .n the pennatal period (POtM^G codes in perinatal chapter of WHO 1992a), apart from low birthweight and asphyxia.
M.itrrmal and Po.i.i,Kai c.iulitions I g
*
i
ch25.qxd 05/24/2005
18:05
Page 10
Table 26.4 DALYs for Perinatal and Maternal Conditioi
ns by Gender, Selected Regions, 2001
(thousands)
World*
Condition
Maternal
South Asia
Sub-Saharan Africa*
All
Male
Female
All
Male
Female
All
26.789
Male
n.a.
Female
26.789
10,069
n.a.
10,069
9.743
3.928
n.a.
1.718
9.743
n.a.
1,718
1.643
5.348
n.a.
1.857
1.643
n.a.
1.857
1,843
1,895
n.a.
742
1.843
n.a.
742
842
n.a.
842
Hemorrhage
3.928
n.a.
Sepsis
5.348
n.a.
Hypertensive disorders ol
pregnancy
1.895
n.a.
Obstructed labor
2.506
n.a.
Unsafe abortion
2,506
1.185
3.507
n.a.
1.185
n.a.
919
3,507
n.a.
1,467
919
n.a.
1,467
1,557
n.a.
1,557
Perinatal1’
90,505
49.384
Low birthweight'
41.117
37.721
43.073
20.442
23,241
17,279
20,047
19,832
11,351
25.015
8.696
13,292
11,723
7.891
14,025
4,501
8,283
3,391
4.957
3,326
9,256
5,195
4.062
7,260
4.423
2.193
2.230
2.899
1,655
1,244
Birth asphyxia (including
birth trauma)
31,972
Other perinatal conditions'*
15,460
17.945
8.198
Swrce WHO
n ’ = noi applicable,
a Excludes lhe island of Mayotte.
c Includes preterm deliXTX"mS
COnfleni‘al SYPhi,iS'aCquired in,ec,ions (respiratory and sepsis), and diarrhea
--^sahcondit.onsori^intheperina^
O neonata mortality, with the greatest average disparity being
nd in Latin American and the Caribbean (http://www
"orldbank.org/poverty/health/).
The former focused on avoidable mortality resulting primarily
from direct obstetric conditions, whereas the latter considered
population risk assessments and highlighted the contribution
of indnect obstetric problems-especially micronutrient
defioencies-and the role for preventive strategies. Clearly, the
choice between different measures of burden has a crucial
influence both on the strategic approach to achieving health
gains and on the prioritization of interventions.
Attributable Burden
hite?5'!"10'1,0" °f n’atCrnal and pcrina,al conditions as part of
.
\
anti much has been written about the prob-
o^rs,al distforhions °f p™ri,,cs (Ab°uzahr
Sadana 2001). Some of those criticisms relate to methods of
s aluation based on disability-adjusted life years (DALYs) espe
' V rCla,'°" 10 d>5“unting and the omission of stillbirths
and others to the inaccuracies and selectivity of the base d^'
on the incidence of complications, on case fatalities and on
.SaharaiiAfr ca ffor the
I Pr"'
5
S0Uth Asia and S“bfocus conditions for 2001. Those two
maXSt aCC0U",|f°r 74 PCrCCnt °f ,hC Sl°bal bl'rd“ °f
The significance of the burden of maternal
and perinatal
conditions is clear from two recent g^bui <
global assessments (CMH
hn
’eled UHTT
002^T”Cr™"05 ,W°
ad“P-d
>.ne
m different conclusions about public health priorkies.
Given the scope and nature of the burden of maternal and peri
natal condmons, no quick fix is available and, thus, no single
intervention warrants exclusive attention. Rather, clusters or
packages of mterventions need to be considered, and this
understanding has long been reflected in maternity services
roug out the world (Milne and others 2004). Even though
the" bas TS Can be CharaCterized or differentiated solely on
the basis of content-namely, the component interventions-
..... ....
Levels and Types of Interventions
Box 26.1 presents one example of a comprehensive strategy for
safe motherhood, ft ihustrates the range of program^
'» I Wendy J GiaHam. John Cai™. Sohinae Shaltactarya. a, al.
I
001-032_ch26.qxd 05/24/2005
18 : 05
Page 11
Box 26.1
Components of a Comprehensive Safe Motherhood Strategy
I he following are part of a comprehensive safe mother
hood strategy:
— screening and treatment for syphilis
antiretrovirals, where voluntary counseling and
testing undertaken, and breastfeeding advice
— tetanus toxoid immunization
— treatment of urinary tract infections
skilled assistance at delivery
care of obstetric complications and emergencies
postpartum care
safe abortion and postabortion services
family-planning information and services
adolescent reproductive health education and services
• community education on safe motherhood and new
born care
• evidence-based prenatal care and counseling
— nutritional advice
iron and folate supplements (multivitamins and
micronutrients)
iodization of edible oils and salt and vitamin A in
areas of endemic deficiency
— blood pressure screening
Source Dayaratna and others 2000
issues raised by maternal and perinatal conditions:
•
•
the scope for both primary and secondary prevention
the difference between the individual receiving specific inter
ventions (here, the mother) and the beneficiary (the baby)
the multiple effects of single (component) interventions on
different outcomes
the multiple benefits to the same outcome of different
interventions
the short- and long-term time frames for interventions and
outcomes
the balance between, supply-side and demand-side
interventions
.
the role for interventions outside the health sector.
maternal death and disability may be avoided by effective,
timely, and appropriate clinical interventions, often referred to
as emergency obstetric care.
Given that complexity and the multiple approaches used to
address maternal and perinatal conditions, no perfect frame
work for categorizing interventions exists. We, therefore, clus
ter the alternative intervention pathways on the basis of the
following three parameters:
+
level ofcarc—home, primary, and secondary
•
time period—pregnancy, labor and delivery, and
postpartum
strategic approach—population-based versus personal
interventions.
1 hree main pathways are available for averting adverse out
comes: preventing pregnancy, preventing complications, and
preventmg death or disability from complications. The first
pathway ,s the only truly primary preventive strategy It
Quality of Evidence
Pregnancy and childbirth have been the subjects of medical
.nvcsfgation for centuries and, indeed, are among the oldest
requ.rcs intervention to avert the occurrence or mistiming of
clinical specialties. As a consequence, a substantial body of opinpregnancy by means of effective family-planning methods, as
ion exists on the signs, symptoms, etiology, prognosis, natural
d.scussed in chapter 57. This preventive approach is relevant
’
history, and management and treatment options for many
for those women who are able to and wish to avoid or delay
.maternal
and perinatal complications, particularly in developed
pregnancy, but it has a limited role for those not in this posi
countries. Much of it can be regarded as conventional wisdom
tion, estimated at between 15 and 57 percent of women age 15
acquired through practice. In contrast, a comparatively small
to 29 (WHO 2002). As concerns the primary prevention of
proportion of interventions can be regarded as based on evicomphcations, comparatively limited reliable evidence is avail
ence, by contemporary scientific standards, and arrived at
able on the true size of the avoidable fraction for many condi
through the conduct of robust research. Thus, in specification of
tions at a population level. The emphasis in this preventive
the content of intervention clusters, a built-in tension exists
pathway ts on maintaining normality and on managing mild
between using the best available knowledge and using only evi
compheatrnns-and thus on good quality of care. Finally,
dence that passes minimum quality criteria. Equally important
Mnlmnal mid PoiiiHitnl Conditions I II
4
I
001-032 Ch26.qxd 05/24/2005 18:05 Page 12
is recognizing the fundamental distinction between knowing
what is effective at an individual case-management level, for
which an evidence base exists for maternal and perinatal condi
tions, and demonstrating effectiveness at the aggregate levels of
composite strategies and entire countries or regions, for which
robust evidence is extremely limited (Graham 2002).
Population-Based Interventions
I he primary aim of population-based interventions is to
reduce the risks leading to adverse outcomes at the population
level rather than at the individual level (WHO 2002).
Population-based interventions are essentially preventive and
seek to promote healthy behaviors, thereby reducing incidence
in the entire population. In the case of maternal and perinatal
conditions, such an approach could be adopted for two major
risk factors: lack of contraception and maternal undernutrition. The grade of evidence for these population-based inter
ventions is primarily level C for the former, but a mixture of A
and B for the latter.3
I erfhty Behavior Change. Fertility behavior is ultimately the
primary sources are available, but there are a variety of mod
eled estimates, such as Praia and others (2004), Walsh and oth
ers (1993), and Winikoff and Sullivan (1987). Model estimates
vary enormously in terms of the size of the effect, depending
primarily on assumptions about the proportion of maternal
deaths caused by unsafe abortion. Investigators estimate the
potential gain from the avoidance of unintended or mistimed
pregnancies to be a 20 percent decrease in maternal deaths in
developing countries (Donnay 2000; Kurjak and Bekavac 2001 •
UNICEF 1999).
Nutritional Interventions. Maternal undernutrition encom
passes two main dimensions: underweight and micronutrient
deficiencies (principally iron and vitamin A). Unlike many of
the direct maternal complications, which are acute at onset and
of relatively short duration, these nutritional problems are
chronic and long term and, indeed, are intergenerational
(Tomkins 2001). The physiological mechanisms by which
undernutrition exerts an influence on outcomes in the mother
and baby are not entirely understood, but a large body of epi
demiological evidence supports associations with, for example,
fetal growth or length of pregnancy (Villar and others 2002)’
Those findings have or
’ ’
' mostly from populations with
originated
either severe levels of undernutrition
--------- or significant cofactors,
such as malaria and other infections.
Considerable uncertainty surrounds the issue of timing
potential interventions, with conflicting opinions about mak
ing targeted interventions during pregnancy; addressing
undernutntion among girl children or adolescents, and apply
primary exposure factor for both maternal and perinatal condrnons. Investigators have shown that the frequency (number
and spacing), the tuning with regard to age, and the desirabil
ity of pregnancy are associated with increased risks, although
some dispute remains about the effect of birth intervals
Researchers have also investigated the influence of those factors
on perinatal conditions, finding dear associations with old or
young maternal age, short interpregnancy intervals, and high
ing strategies for women of reproductive age, including perior first birth order, with many of those variables also being
conceptual women (Gay and others 2003; Rush 2000). Further
interrelated (Bale and others 2003).
6
ebate relates to the use of supplements versus food fortifica
hack of effective use of contraception may result in
tion.
A systematic review by Villar and others (2002) of ranunwanted or mistimed pregnancies. Unintended pregnancies
are kno'™ to be associated with adverse maternal outcomes, . domized controlled trials to prevent or treat adverse maternal
outcomes and preterm delivery concludes that limited evidence
in Ind,ng unsafe abortion. Contraceptive behavior is clearly
supports large-scale interventions with multivitamins, miner
c turmmed by a host of socioeconomic, cultural, religious, and
als, or protem-energy supplementation, but that iron and folic
med'cal factors (Hussain, Fikree, and Berendes 2000; Marston
acid are effective against anemia. Rouse (2003) emphasizes the
nd Cleland .003; Mwageni, Ankomah, and Powell 2001),
potential cost-effectiveness of vitamin A or beta-carotene sup
'Inch also have a bearing on intervention options. Most of the
plementation in reducing maternal mortality if the findings of
options on the demand side focus on information, education,
West and others (1999) from Nepal are replicable elsewhere.
commun.cat.on; those on the supply side focus on clientfr.endly serv.ces. At a macro level, those intervention options
have been cred.ted with the substantial increase in contracep
Personal Interventions
tive use n. developing countries over the past 40 years, which,
When we consider interventions directed at individuals rather
fer I'it"' '77’ a7 COn,ributor ,o "’c overall fall in the total
than whole populations, the need for a continuum of care for
r .l,t) rate from 6 to 3 (Cleland and Ali 2004). Nevertheless, a
T be*' a"d baby 1,1 terms of time (before and after delivery),
•%n luant unmet need for contraception persists in many
P ace (in ing home and health services through an effective
developmg countries, with high levels of unsafe abortion as a
re erral chain), and person (the provider of care) is important
proxy indicator of that need.
variety of conceptual frameworks emphasize this continuum
As regards evidence of the effectiveness <
in explicitly reducing maternal mortalityof family planning
nd the dangers of fragmentation. Care to prevent or treat
' or disability, no
w vast majority of maternal and perinatal conditions can be
12 i Wendy j Graham. John Cairns. SohineeBhatl3Charya.el al
I
LCP 001-G32 ch26.qxd 05/24/2005 18:05 Page 13
provided at home, at the primary level (clinic or health center),
and at the secondary level (district hospital),'' with the district
hospital or equivalent regarded as the essential planning unit
for service delivery (WHO 1994). This system is comparable to
the “close-to-client” health system that the Commission on
Macroeconomics and Health (CMH 2002) has proposed,
whereby trained staff members other than doctors provide
much of the care, with an emphasis on primary prevention and
management of acute conditions.
Home-Based Care. Two topical interventions that fall into the
category of home-based care are (a) information, education,
and communication and birth preparedness and (b) male
involvement (for home-based newborn care, see chapter 27).
Evidence in this cluster of interventions falls predominantly
into the level C category.
B.rth Preparedness Many descriptive studies indicate that
women, relatives, and other members of the community fre
quently do not recognize danger signs in pregnancy, childbirth,
or the puerperium, and that lack of recognition can have seri
ous consequences for mother and baby (Gay and others 2003).
Health education interventions at prenatal clinics appear to
be less successful at raising awareness and increasing the use
of emergency obstetric care than the use of pictorial cards
(Khanum and others 2000) or community education (Bailey,
Szaszdi, and Schieber 1995).
X
Birth preparedness includes planning for the place and the
health center or hospital, whenTeX^l^d^Zlim^identi’
fymg a compatible blood donor in the case of hemorrhage
(Portela and Santarelli 2003). Initiatives to promote birth pre
Primary-Level Care. Primary-level care is widely regarded as
the crucial entry point to maternity services - and also to care
before and after pregnancy. The focus here is essentially pre
ventive, but with the capacity to detect problems, to manage
mild complications appropriately, and to stabilize and then
refer cases that require higher-level care. Although the name
used for primary care facilities varies from country to country,
we employ the commonly used term health center. In terms of
functionality in relation to maternal and perinatal care, the
health center should provide prenatal, delivery (including
management of complicated abortion), and postpartum care
(including family planning and postabortion counseling), as
well as care of the newborn.
The management of complicated cases is usually discussed
at two levels: basic emergency obstetric care (BEmOC) and
comprehensive emergency obstetric care (CEmOC), the dis
tinction being made on the basis of the number of signal or
essential clinical functions performed.5 This distinction forms
the basis of a set of process indicators that the United Nations
(UN) has endorsed for program monitoring (UNFPA 2003).
The capacity of health centers to provide BEmOC depends on
t le availability of supplies, drugs, infrastructure, and skilled
providers. Some of the signal functions may not always be per
formed by midwives or nurses, sometimes because of the regu
lation of roles by the government or professional bodies. For
t is reason, a further distinction can be made between full
BEmOC, which comprises six functions, some of which may
require a doctor, and obstetric first aid, which includes two sig"hl a^0"5 UniVer;ally Performed by midwives
5 and nurses:
the
’ administration
dm'nlStra,10n of antibiotics
ail“biotics or
' '
oxytocics, intravenously or
intramuscularly.
pare ness can clearly be home or community based, but studes have emphasized the importance of linkages with prenatal
are so as to mclude appropriate recommendations for inla-
P rtum care (Shehu, Ikeh, and Kuna 1997). In circumstances in
triradmonal
di i ‘"TlbirthrrV
'CCS arc ',f |H>Or <|,,alily "r arc l"’d«used,
attendants, or relatives are often the only
S*’* ,hUS’
Male Involvement Many studies have observed positive benefits from the involvement of male
behavior related to j---------__ _ .is now aavocated
,
7 ' ’ dIIU oincrs
2003). That involvement
----- 1 is now advocated as an essential
art I,ovules one of the rare examples of robust assessment of
a mterventmn package (Villar and others 2001). As Bale and
Others (2003) note, even though many of the component clin-
■ca ■"'e-ntionsare effective in terms of perinatal outcomes
(Bergsjo and Villar 1997), reliable evidence of an effect on
maternal mortality in developing countries is not available
, i.v
.
.. ......... early detection is followed
___prenatal
_____• care does seem to reduce
. by apDronn.itp
appropnate treatment,
adverse outcomes from specific maternal conditions, including
22003).
oZm M H°? Making PrC8nan^ Safcr
(WHO h pertensive disorders of pregnancy, urinary tract infection
Models and mechanisms for achieving this involvement
controversy
Xr d ' PreSCn,a,i°n5 (Carroli> Roo-y, and Villar 2001;
have not been robustly evaluated, and considerable c—
concerns those that are based on behavioral and soc
cognmve theories that presume lack of knowledge a'sSocial
’ihe
2000)Pr
P°r,ela a"d San,ardli 2OO3; Raiu and Leonard
, eT;0 '997)- C°nVerSely’tha baited effectivenes
of prenatal risk screening at a population level is now w.ddy
acknowledged (Graham 1998), The poor predictive value of
many screening tools for maternal complications reinforces the
importance of access to emergency obstetric care for all women
Malomol ond Perinalol Conilliions I 13
I
I
0J2 ch26.qxd 05/24/2005
18:05
Page 14
who develop a need for it and underlies calls for skilled atten
dance at all deliveries. Many health experts accept screening
and treatment for syphilis and immunization with tetanus tox
oid as important prenatal interventions (Bale and others 2003).
Similarly, the prevention and treatment of anemia and of
malaria, with prophylaxis or bednets, are widely regarded as
essential elements of routine prenatal care. Nutritional supple
mentation, however, remains more controversial.
Prenatal care has been assessed not only in terms of content,
but also in relation to alternative models of the number and
timing of visits (Munjanja, Lindmark, and N
Nystrom 1996).
Strong evidence exists on the cost-effectiveness of a targeted,
four-visit schedule (Villar and others 2001) that includes an
educational clement on the recognition of danger signs and the
use of skilled attendance at delivery.
The principal sources of international data on levels, trends,
and differentials in prenatal care coverage are the DHSs. The
I.Hest statistics show comparatively high coverage levels
When measured in terms of one or more visits-levels average
71 percent for Sub-Saharan Africa—but comparatively little
improvement between 1990 and 2000. Within countries wide
socioeconomic differentials in uptake are apparent.
grade evidence supports a number of clinical interventions,
such as active management of the third stage of labor, as well as
essential newborn care.
Once again, the principal sources of data on levels and
trends in coverage of skiUed attendants at delivery are the
DHSs. The data, however, are based on womens self-reports of
who attended their deliveries, include only live births, and have
major definitional uncertainties. Some countries, for example,
use terms such as supervised deliveries and include as attendants
koth auxiliaries and trained traditional birth attendants (sec
Bell, Curtis, and Alayon 2003 for a critique of these data). A
global analysis of trends in deliveries by skilled attendants
showed wide variations in progress across different regions,
with the latest figures for Sub-Saharan Africa, Asia, and Latin
America and the Caribbean for 1990-2003 being 48, 59, and
82 percent, respectively (AbouZahr and Wardlaw 2001;
WHO 2004a). The proportion of deliveries with health profes
sionals present (doctors, midwives, nurses) is one of the proxy
indicators for the MDG on maternal health (Graham and
Hussein 2004). It demonstrates not only major differentials
between countries, but also wide variation in uptake across
socioeconomic groups within countries (De Brouwere and Van
Lerberghe 2001). Although skilled attendants do not necessar
ily operate only in fixed health facilities such as health centers,
the DHS data show low levels of professional attendance in the
Delivery Care As indicated earlier, the risks of adverse out
comes in mother and baby are usually highest during the intra
partum period. Even though health experts have long appreci
community. Promoting skiUed attendance is thus essentially
ated this fact, prioritization of this element of safe motherhood
advocating for institutionalizing deliveries.
is comparativdy recent. Much has been written both on this
shift m emphasis and on the underlying rationale, as well as
Postpartum Care Primary care services continue to neglect
on what skilled attendance at delivery should comprise (De
the postpartum period despite significant morbidity among
Brouwere and Van Lerberghe 2001). Investigators
have suggest- *’ mothers and babies during this time. Routine performance of
ed a variety of conceptual models for defining content, with
postnatal checks is not widespread, and most contacts with
varymg degrees of emphasis on the attendant and
on the
services after delivery tend to focus on educational messages
enablmg environment (Bell and others 2003). All these models
recognize that skilled attendance encompasses both normal ’ <>n, for example, danger signs, breastfeeding, nutrition, and
lifestyle.
and comphcated deliveries, with the focus on the former and
O” lhe management of mild complications at the primary level
as ts consistent with BEmOC, and with referral to CEtnOC ai
Inc secondary level when necessary.
Key unresolved issues at the primary level relate to the skills
and scope of work of the attendant, especially in relation to
being a mult.purpose health worker, and to the potential role of
nonprofessionals, such as auxiliaries and trained traditional
brnth attendants (But,iens, Marchal, and De Brouwere 2004).
Work by Kobhnsky and Campbell (2003) has helped to inform
this debate by proposing four basic models of delivery care that
vary accordmg to configurations of place of delivery and atten
dant. Ev,deuce on the effectiveness of the alternative models at
a populatton level is lacking, and support for skilled attendance
at debvery is, thus, based primarily on historical and contemP-ary ecological analysis (De Brouwere and Van Lerberghe
MO!, Van Lerbergheand De Brouwere 2001). Conversely, high-
Postaborlton Care One significant area of service delivery that
does not fit well with descriptive frameworks based on prena
tal, intrapartum, and postpartum care is the management of
comphcated abortions. Unsafe abortion accounts for a signifi
cant proportion of the burden of maternal conditions, but it is
Still treated as the poor relation in the debate on intervention
strategies (De Brouwere and Van Lerberghe 2001). In particu
lar, with the prioritization in recent years of skiUed attendance
at delivery, both the service base for and the provider of '
postabortion care have become less well defined (Dayaratna
and others 2000). This crucial element of obstetric care faUs
into BEmOC in the case of mild complications and CEmOC
tor more serious cases, but whether it is regarded as part of
prenatal, delivery, or postnatal services appears to vary from
setting to setting. Moreover, postabortion care illustrates the
dangers of the fragmentation of broader reproductive health
» I Wend, J Graham, John Cairns. Sohiriee BhaUacharya. e( al.
I
■ <.
05/24/2005
18:05
Page 15
(p
care, because primary prevention and counseling after treat
ment for complications tend to fall within the remit of family
planning services, whereas emergency care at the primary level
is usually provided as part of maternity services and at the sec
ondary level may fall within obstetrics or gynecology services.
Secondary-Level Care. Secondary-level care is hospital-based
care, generally at the district level, including CEmOC. Because
it occurs by referral, this level of care needs to be linked to the
primary
primary level
level in
in an
an effective
effective chain
chain of
of communk7tion7(Murrav
communications (Murray
and others 2001). The focus al the district hospital is on secon sccondary prevention, with the ability to manage the principal
maternal and perinatal conditions discussed earlier- thus
district hospitals must be able to provide surgical interventions
and the requisite backup, such as blood banks (Kusiako
Ronsmans, and Van der Paul 2000). In many countries’
however, the district hospital is also the local provider of pre ’
ventive services, including prenatal and normal delivery care- as
such, it is responsible for attending to a wide mix of uncomplicated and complicated cases.
Although no high-grade evidence of the effectiveness of
CEmOC is available, many health experts agree that maternal
mortality cannot be significantly reduced in the absence of
such• care
(
-e (Bale
and others 2003). The issue thus becomes one
of the cost-effectiveness
of otli
• "
---/-.her
strategies, given the presence
of CF.inOC. Th<IC UN agencies have endorsed the threshold of
y per 500,000 people. Data indicating the
one CEmOC
CF.„,C,C /acilit
faculty
attainment of this ratio-and, indeed, the percentage of met
need for CEmOC-are not widely available. Similarly, reliable
information on geographic or socioeconomic differentials in
signatory countries. However, as observed at the ICI’I) 4- It)
Conference, many promised changes remain at the level of pol
icy pronouncement and have not yet been implemented. The
stagnation is most notable in relation to maternal mortality
and the HIV pandemic, especially in Sub-Saharan Africa. The
failure to fully implement the ICI’I) consensus can be attrib
uted to lack of political will, inadequate funding for programs
to further reproductive health, and weak health systems. It is
03,47
^e’8"
°f
MDG Procla,nati«»
(,oha,1SSOn and Stewart 2002)’ although it could well suffer the
i1’*1! ,
U.n CSS SpCdal a,lention is
maternal and child
n i -H ’
C°nleXt °f Scclorwidc approaches and Poverty
u » uttlon Strat^X Papers (UNFPA 2003). Some suspect that
f°
’T niodaht,CS
not Eive reproductive health the
focusV™
and attention
t..;; needs m.
atte,lt,°nit
't requires,
rcM»'rcs. because
because co!..
competing
may
crowd
out.’’ °
Others
argue,’however,
sectorwide approaches
approach,
i it T
thCrS ar8UC
howevcr’that scclorwidc
can be "a b .‘r f - ’naternal
■
* •hea,th
■ • bccause
they offcr a
effective platform
for
addressing
^^7" f<7 ,add.rCSSin8 ai’ing hcal,h systcms
(Goodburn and Campbell 2001).
Whether at the national or international level, advocacy for
maternal and perinatal health should focus on the following
seven key message areas:
.
.
.
*
consequences of not addressing maternal and perinatal
health
•
■
access to CEmOC is extremely limited.
Policy Considerations and Approaches
The health of mothers and babies is a human right and necrls
be underpttmed by policies and law, that increase access to
nformatmn and good-quality, affordable health services
(Germam 2000). A positive policy environment is crucial for
p™>’»>'ng maternal health and reducing the burden of mater" ' I ’’"'IT COI’di,i'-P«hcy considerations need
f, > xyond the health sector to include related issues, such as
ransportatton, nutrition, girls' access to education, and gender
b.ases in the control of economic resources. Through a human
nghts based approach, programs can be fashioned to ensure
'
W°man has lhc eight to make informed decision,
about her own health and has access to quality services bZ
during, and alter childbirth (Freedman 2(101)
I be ICPD marked a dramatic shift not only by putting lhe
...
Zn" n'
PrOblCn' ,
r
• dlucncing maternal and perinatal outcomes
tionswor^
hCalth Pro8ran,s and w,"ch interven-
costs of improving maternal and perinatal health
responsibilities at each level of the health system and beyond
pohey and legal impediments to implementing comprehensive safe motherhood and newborn health programs.
nrsd the Hea thy Newborn Partnership, seek to promote mater
nal and newborn health at the global level. Their purpose is to
create awareness by changing the language of discourse, build
i"g“if
mteniational political commitment, developing global
l"’Provil'8’, access to technical information for
PrOVlderS
Pl'<,«li'"’llM
- i,,,d program
managers.
COST-EFFECTIVENESS OF SELECTED
INTERVENTION PACKAGES
i,’tro-
Cost-effectiveness analysis (CEA) faces several major chal-
i>D ,Hn
'C,'Ve
"‘h Paradigm- The firS' d-de of
c IQ D plan of action was marked by major improvements
P Kies related to maternal health in most of the 179
of m ,7 I rCSuCC‘ '0 eValUating tl,C PreVCn,i°” ai’d '^''’’ent
Of maternal and perinatal conditions. First is the sheer range of
onditions and potential interventions. The breadth of the
clinical area imphes the need to make tough choices with
ducinTthe "^d
S,a8C’ b"‘ als0
Malciii.il niiil l’niiiial;il Comlilinns I If,
-I-
I
-032 ch26.qxd 05/24/2005
18:05
Page 16
respect to which packages of interventions to compare. A sec
ond and related challenge is the lack both of reliable data on the
burden of conditions and of high-grade evidence on the effec
tiveness and costs of packages. As a result, we can assess only
the relative cost-effectiveness of different packages of interven
tions by means of modeling. Thus, the third set of challenges
is associated with modeling, which makes the analysis vulner
able to all the usual criticisms of the modeling of costeffectiveness—in particular, uncertainly about the direction of
any b.as introduced and the difficulty of establishing the valid
ity of the model (Sheldon 1996). Finally, there arc the related
issues of the appropriateness to maternal and perinatal condi
tions of standard outcome measures used in the model—in
particular, DALYs, which exclude stillbirths and indirect mater
nal conditions (AbouZahr 1999; De Brouwere and Van
I.erberghc 2001).
others 2003), we evaluated intervention packages with respect
to a counterfactual (base scenario), varying the content and
coverage. We also performed sensitivity analyses to examine the
effects of changing the values of key variables for costs, effec
tiveness, or both. Each intervention package scenario specifies
different dimensions of prenatal and intrapartum care provid
ed at primary and secondary care facilities. As regards the
assumed pathways through which women with normal or
complicated pregnancies may or may not access care, the cru
cial entry point in our model is prenatal care. That choice influ
ences the detection and treatment of mild and severe compli
cations during the antepartum period at both the primary and
the secondary levels, as well as the proportion of women deliv
ering with a health professional present and with improved
access to emergency care for intrapartum or abortion-related
complications. In our CEA model, these effects are achieved
primarily through two types of interventions:
Selected Intervention Packages
• improvements in the quality of care, incorporating the tech
For some of the reasons mentioned in the previous subsection,
nical content or the proportion of women in receipt of the
researchers have made few attempts to model packages of inter
care needed (that is, met need)
ventions for maternal and perinatal conditions, and many of
• increases in the coverage of care—namely, the proportion of
those attempts do not specify content in sufficient detail to
women accessing care.
repheate the package. Our approach is to define content by
beginning with a literature search of best practices in prevent
Routine prenatal care can be characterized in terms of
ing and managing the focus maternal and perinatal conditions
whether it is a basic or an enhanced package—in other words,
acknowledging that, by excluding conditions that impose a
its technical content (table 26.5)—and by the percentage of
lesser burden, we ignore interventions that might be highly
women
accessing the package-in other words, its coverage.
effective and cost-effective. We then grouped those interven
Delivery at a primary-level health center is viewed as having a
tions that are considered effective and that are either being or
hkely to be tmplemented on a substantial scale into packages of s single quality dimension in terms of content—namely, whether
BEmOC is available for women who develop mild complica
care bearing m mind previous CEA work, such as the WHO
tions, including complicated abortion (table 26.5). BEmOC is
mother-baby package (WHO 1994). Expert panels then
assumed
to require the presence of a doctor at the health cenreviewed the component interventions and the packages and
assisted With identifying resource requirements. Given the i ter; otherwise, only obstetric first aid is presumed to be availcomplementary CEA elsewhere in this volume on interventions ' able, covering just the two signal functions described earlier.
A percentage of women with severe complications who
relevant to maternal and perinatal conditions such as family
access primary care will go on to secondary care. This percent
Planning, we focus on care during pregnancy, postpregnancy
age is assumed to be 20 or 50 percent of complicated cases
care, and care immediately postdelivery-in other words, on
attending primary care. Our model makes no provision for
clusters or packages of interventions typically referred to as prewomen who access secondary care directly in the event of a
n..lala,re. del, very or intraparu,,,, care, and emergency obstetric
serious complication, although it does allow for those who
care. Table 26.5 outlines the content of those packages
were attending the hospital as their local provider of primary
When one considers the intervention packages, contextual
care. Of those women who access the secondary care facility
actors are clearly crucial. Given the particularly high burden in
from the primary level, a proportion will receive the CEmOC
- >uth Asia and Sub-Saharan Africa, we chose those two regions
t at they need (assumed to vary between 50 and 90 percent of
•>1 the specific health system scenarios for this chapter. Those
complicated cases that reach secondary care). This figure
regions are also characterized by high levels of poverty and
reflects such issues as staff skills and motivation and the availencompass some of the most heavily indebted countries in the
ability of drugs and equipment. For the other quality-of-care
element—namely, the technical content of CEmOC-we
consider two levels: with (enhanced package) and without
Comparison of Alternative Intervention Package Scenarios.
base package) selected interventions for high-risk babies
Mlowmg the approach of generalized CEA (Hutubessy and
(table 26.5).
16 i Wendy J Graham. John Cairns. Sohmee Bhaltacharya, el al.
I
r 001-('32 Ch26.qxd 05/24/2005 18:05 Page 17
*
Table 26.5 Care Packages at the Primary and Secondary Levels
Level of care
and condition
Routine prenatal care
at the primary level'1
Content
Base
package
Enhanced
package
V
y)
Clinical examination, including for severe anemia, height and weight, blood pressure
Obstetric examination lor gestational age estimation and uterine height, fetal heart, detection
ol malpresenlation and position, and referral
Gynecological examination
yl
Urine test (multiple dipstick)
J-abora'ory tests, hemoglobin, blood type and rhesus status, syphilis and other symptomatic
testing for sexually transmitted diseases
Advice on emergencies, delivery, lactation, and contraception
Education about clean delivery, warning signs, and premature rupture of membranes
y/
yJ
V
y/
Multivitamin supplementation
'J
>1
Tetanus toxoid immunization
n.a.
y/
y/
y/
HIV voluntary testing and counseling
Antimalarial chemoprophylaxis in endemic areas
Screening and treatment for syphilis
n.a.
yl
n.a.
J
Balanced protein-energy supplementation for all women
“Clean delivery technique, clean cord cutting, clean delivery of baby and placenta--------------
y/
yl
n.a.
V
Active management of the third stage of labor, including oxytocics
T
7
y/
yl
Episiotomy in appropriate cases
yl
V
yl
yl
y/
yl
Recognition and first-line management ol delivery complications (for example obstructed lab.
)or.
cesareand live™^alOr,Pe'''bdlSpr0[Wrli°n' malp°sili°" a"d malpresenlation. previous
cesarean delivery, postpartum hemorrhage, and preeclampsia or eclampsia) and referral
Intravenous fluid
Intravenous uterotonics, if bleeding occurs
Partograph
Essential newborn care
Intravenous antibiotics
Magnesium sulfate
*
yj
yl
y/
V
yl
yl
yl
yl
Forceps or vacuum extraction
yl
Manual removal of placenta
V
Removal of retained products of conception \
yl
Corticosteroids for preterm labor
yl
Antiretrovirals for prevention of mother-to-child transmission of HIV
V
Antibiotics lor premature rupture of membranes
yl
CEmOC package at the secondary lever
Postpartum hemorrhage
>/
yl
y)
Iron and folic acid supplementation
Delivery care at the
primary levelb
J
y)
Recognition of high-risk
cases and arrangements for delivery in a facility
Grouping of blood
Iron and folate supplementation
Blood transfusion
>/
J
J
Uterotonic drugs, oxytocics
>1
V
V
yl
Bimanual compression of uterus
yJ
yj
Manual removal of placenta
>/
yl
Uterine packing or balloon tamponade
y/
Fluid replacement
y/
Hysterectomy
yl
yl
Removal of products of conception
yl
yl
V
yl
yl
ssxs.-s:1.
S. removal of products
yl
yl
yl
(Continues on the following page.)
Maternal and Perinatal Conditions I 17
I'CP 001-0?2 ch26 .qxd 05/24/2005
18:05 Page 18
Table 26.5 Continued
Level of care
and condition
Antepartum hemorrhage
Base
Content
package
Enhanced
package
a/
a/
a/
a/
a/
a/
a/
a/
a/
a/
Early detection of major placenta previa and abruption.
Grouping and saving blood
Iron and folate supplementation
Cesarean section for major-degree placenta previa, abruption with a live baby
Blood and fluid replacement
Oxytocics
Sepsis
Antibiotics for premature rupture of membranes, cesarean section
7
Fluid and blood transfusion
Intravenous antibiotics
Evacuation of products of conception
Drainage of abscess
-Treatment of shock with fluids or blood, nitroglycerine
Pregnancy-induced
hypertension
Early detection and management of preeclampsia
Calcium supplementation in high-risk cases
a/
>/
a/
a/
a/
a/
a/
a/
a/
a/
7~
a/
a/
Aspirin to prevent preeclampsia
V
Intravenous magnesium sulfate
Antihypertensive drugs to reduce blood piessure
Immediate delivery if more than 36 weeks
Magnesium sulfate and antihypertensives for postpartum eclampsia
Obstructed labor
a/
a/
Antioxidants to prevent preeclampsia
a/
a/
a/
V
a/
a/
a/
- -------
Partograph
a/
a/
I
V
Cesarean section
Symphysiotomy
V
V
V
Destructive operation
♦
Antibiotics
Fluid and blood transfusion
Hysterectomy
Abortion
Evacuation of retained products of conception
V
\
Fluid or blood transfusion
7
v
Postabortion contraceptive advice
>/
Intravenous antibiotics
Ectopic pregnancy
1
y/
J
>/
a/
7
a/
a/
a/
Proof puncture (culdocentesis)
Laparotomy and salpingectomy
Blood transfusion (autotransfusion)
High-risk infant
>/
Forceps or vacuum extraction11
Corticosteroids for preterm labor
'
Antiretrovirals for prevention of mother to child transmissibn of HIV
Antibiotics for premature rupture of membranes
Source Aulhors.
— noi available
'
*-------------
v
7
v
V
J
..............
18 I Wendy J Graham. John Cairns, Sohinee Bhattacharya. et al
I
|DCP_001-032_ch26.qxd 05/24/2005 18:05 Page 19
1 he base case for our CEA model assumes the following:
basic technical content for the prenatal care package
prenatal care coverage for 50 percent of pregnancies
only obstetric first aid (two signal functions) available in
health centers
• 20 percent of women with severe complications accessing
secondary care
• 50 percent of those severe cases receiving the CEmOC that
they need.
•
•
The different assumptions regarding quality of care and
coverage can be combined in many different ways, yielding a
large number of potential packages and a larger number of
potent,al comparisons between those and the base package
However, not all possible scenarios are meaningful. For exam
ple because the base prenatal care package does not screen for
IV, matching that package with enhanced delivery care that
prov.des antiretrovirals to reduce vertical transmission would
^ -appropriate. We identified six packages for comparison
vtth the base case, representing a range of safe motherhood
strategies and focusing on prenatal and delivery care. Table 26 6
summanz.es these alternatives and indicates their essential
characteristics from a safe motherhood perspective.
Resource Use and Costs
We adopted an ingredients approach (Creese and Parker 1994)
to tdentify resource use. For this type of bottom-up costing, we
prepared fists for prtmary- and secondary-level care facilities of
types of personnel, drugs, supplies (medical and nonmedifal)
medteal and surgical equipment relevant for thc interventions
and cap,tal ltems (vehicles. buildings> buildi
J
th Xn
/' °Ur ,deniifica,ion of resources was based on
WHO mother-baby package costing tool (WHO 1999) with
necessary modifications because of the content of care pack's
d.ca ed m table 26.5. We estimated the costs for clinkal peronnel on the basis of salaries for different grades according to
he gu.dehnes provided by thc volume editors for the two
for e ch cZ°nSt' ThC ,imC reqUirCd
diffCrent Staff mcmb-s
or each care mterventton and thc changes in time and personnel because of varying content and coverage of packages were
m ormed by expert panel reviews, and we then calculated the
costs. We valued the other nontraded inr
‘ using
’ information
■ ■puts
primarily provided by WHO-CHOICE (20047 ~
. Cost-Effectiveness Ratios
to anmx"^ A?
Va™ble aSSUmpti°the red Kt 1, J m°St important assumptions concern
he reductble burden of these conditions, the effectiveness of
the interventtons, and the availability of appropriate human
resources. We have assumed that increases in care can be
achieved without major capital investments and that human
resources are not in short supply; therefore, more could be used
(with given wage rates) as required for increased activity and
enhanced coverage.
Table 26.7 summarizes the findings of the CEA in terms of
incremental cost-effectiveness ratios (ICERs) for the six pri
mary comparisons between the base scenario and alternative
intervention packages for a population of 1 million. Table 26.8
givesi details of total costs, deaths averted, life years saved, and
DALYs averted. Table 26.9 shows the findings of the sensitivity
analysis in terms of how the ICERs change when different
assumptions (see annex 26.A) are made with respect to effec
tiveness, met need, and inpatient costs.
In interpreting the results, note that they
are point estimates. Even though they are based on the best information
currently available, all the inputs into the model are subject to
some degree of uncertainty. Without access to robust data on
individual costs and effects or without specifying distributions
for each variable, it is impossible to identify confidence limits
for the esumated ICERs. Thus, we do not know, for example,
e er tie difference in the incremental cost per DALY
averted for Sub-Saharan Africa between increased coverage at
Pn-ry leve! (US$92) and improved quality of CEmOC
(US$151) reflects a genuine difference in cost-effectiveness
^267“ ‘here are °Verlappin8 confidence intervals
With those important caveats in mind, at first sight the
residts for South Asia and Sub-Saharan Africa appear quite difrent. For each intervention package, regardless of the specific
assumpt.ons made, the cost per DALY averted is always lower
m Sub-Saharan Africa. The higher costs of care in Sub-Saharan
fnca see annex 26.A) are thus more than compensated for by
dre higher effectiveness, which is a result of the region's greater
burden. However, some important similarities are apparent
between South Asia and Sub-Saharan Africa. Leaving aside
mem’) t a"d|57,hC °PtiOnS Wi,h°Ut
-pplernents), the results tor both regions show a consistent pattern
Improvements in the overall quality of care, especially at the
prtmary level through the provision of BEmOC (option 3a)
nos! coTei? i;’CreaSCd 0VCra"
(°Pti°" 5a>'
the
“
packages-and both include
They/are followed by increased cover.
.age at the prtmary level (option 2). Improved quality of
CEmOC (opnon 4) ,s the least cost-effective option. Removing
Utrmonal supplements from the packages makes relatively lit
effe
n“>m Ub’Saharan AfriCa’ Sli8h“y ‘"-easing cost
effectiveness, but tn South Asia, options 3b and 5b become less
ex , ™ t
expki^
|”
,,es
nUtri,i°nal -PP'^ems removed. The
thc ICERs of nutr.t.ona| s i|cmcnis
US l’ 10 in rh 5T8 ” US$45
SOU,h Asia a"d USSI <8
US$110 in Sub-Saharan Africa, depending on whether the
Miilomiil.wIPufindialCondHfons I 19
I
o
f
■5
5
t
i
i
I
f
I
53
Table 26.6 Comparisons Undertaken for CEA
2
Primary level
Abbreviated
description
of package
Option
number
Routine maternity
care
Base
Coverage
Quality of care:
technical
content
Secondary level
Coverage*
Quality of care:
Percentage receiving
care neededi
Quality of care:
technical content Interpretation
Safe
motherhood
strategy
Resource
implications
50 percent of
pregnant women
attend prenatal
.carer 50 percent of
pregnant women
have professional
intrapartum careb
See first two
panels of
table 26.5
20 percent of
complicated cases
at the primary
level referred to
the secondary
level
50 percent of those
reaching the secondary
level receive the CEmOC
needed
See table 25.5t
Basic package of
prenatal and
delivery care
Content of package
essentially the same
as WHO mother-baby
package, plus
magnesium sulfate
and active manage
ment of labor
Costs typical of
WHO mother
baby package
Increased primary- 2
level coverage
70 percent prenatal
care; 70 percent
delivery care
No change from
base
No change from
base
No change from base
No change from
base
Benefit from
increasing coverage
Information, educa
tion. and communica
tion for increasing
uptaxe of prenatal
and delivery care
Costs of informa
tion, education,
and communica
tion; increased
personnel; drugs
Improved overall
quality of care
with nutritional
supplements
3a
No change from
base
Enhanced prenatal
and delivery care
(BEmOC)
No change from
base
70 percent
Enhanced CEmOC
Benefit from enhanc
(adds interventions ing quality (content
for high-risk babies) and receipt of care
needed) at the primary
and secondary levels
Prrrision of BEmOC
at T.e primary level
Costs of doctors
and equipment at
the primary level,
training for
BEmOC and
CEmOC. costs of
BPS
Improved overall
quality of care
without nutritional
supplements
3b
No change 'rom
base
Enhanced prenatal
and delivery care
(BEmOC) without
BPS
No change from
70 percent
Enhanced CEmOC
As for 3a without
(adds interventions nutritional
for high-risk babies) supplements
As "or 3a
As for 3a without
costs of BPS
base
o
KJ
Improved quality
of CEmOC
Improved overall
quality of care and
coverage with
nutritional
supplements
Improved quality
and coverage
without nutritional
supplements
4
5a
5b
No change from
base
70 percent prenatal
care; 70 percent
delivery care
70 percent prenatal
care; 70 percent
delivery care
No change
from base
Enhanced prenatal
and delivery care
(BEmOC)
Enhanced prenatal
and delivery care
(BEmOC) without
BPS
No change
from base
50 percent
50 percent
80 percent
90 percent
90 percent
No change
from base
f
I
f
Improved quality
of CErnOC
Cost of additional
personnel time
and drugs
n,
KJ
cn
x
Q.
o
Enhanced CEmOC
Benefit from improved
(adds interventions quality (technical con
fer high-risk babies) tent and percentage
receiving care needed)
and coverage at the
primary and secondary
levels
Comprehensive
package: improved
coverage and content
with BPS
Enhanced CEmOC
Benefit from improved
(adds interventions quality and coverage
for high-risk babies) at the primary and
secondary levels
without BPS
Improved coverage
and content without
BPS
Source. Authors.
___
«
BPS = balanced protein-energy supplementation.
a. Defined in terms of the percentage of complicated cases at the primary level refered to and reaching the secondary level.
b. Includes obstetric first aid for complicated cases, including aoortion and postpartum complications.
c. The seconoary level will also provice some prenatal arxt delivery care for normal cases, as defined in the first two panels of table 26.5 for the base oaoage at the primary level.
I
Benefit from increased
percentage of women
with severe complica
tions receiving the
CEmOC needed
Costs of
providing and
running ambu
lances. costs
of additional
personnel and
drugs, training
for BEmOC and
CEmOC. costs
of BPS
KJ
$
o
ui
CD
$
V
0>
Ifl
<0
As for 5a without
the costs of BPS
-
0)1-032 Ch26.qxd 05/24/2005
IC;ERS Per
fu.o. uoiiarsi
Option
number
18:05
Page 22
P°Pulati°n' s°u‘h Asia and Sub-Saharan Africa
Alternative compared with
the base package
2
Increased primary-level coverage
3a
Improved overall quality ol care with
nutritional supplements
3b
Incremental cost per
Incremental cost per
death averted
Incremental cost per
life-year saved
DALY averted
South
Asia
Sub-Saharan
Africa
South
Sub-Saharan
Asia
Africa
South
Asia
6.129
3,337
217
119
148
5.017
92
2.729
165
90
142
83
8.975
2.538
296
84
240
7/
10,532
5,089
372
195
255
5,297
151
2,915
177
98
144
86
2.865
269
96
203
84
Improved overall quality of care
without nutritional supplements
4
5a
Improved quality of CEmOC
Improved overall quality of care and
coverage with nutritional supplements
5b
Improved overall quality of care and
coverage without nutritional supplements
7.944
Sub-Saharan
Africa
Source Authors' calculations
°f '"tervent.on Packages per Mi||ion Population, South Asia a(
Option
number
Total costs
(US$)
Intervention package
South Asia
1
Routine maternity care
2
Increased primary-level coverage
3a
Improved overall quality of care with nutritional
Improved overall quality of care without nutritional
4
Improved quality of CEmOC
5a
averted
saved
averted
Percentage
of DALYs
averted that
are maternal
79
2,240
3.273
50
111
3.136
4.582
50
163
4,793
6.225
26
118
3.415
4.727
35
420.918
80
2.272
3.320
1.2^7.354
50
245
7.201
9,354
26
177
5.131
7.103
35
'
Improved overall quality of care and coverage with
nutritional supplements
5b
Number of
DALYs
408.976
757.433
supplements
Number of
life years
603.071
829.505
supplements
3b
Number
of deaths
Improved overall quality of care and coverage without
nutritional supplements
1.186.123
Sub-Saharan Africa
1
Routine maternity care
2
Increased primary-level coverage
3a
Improved overall quality of care with
nutritional supplements
3b
Improved overall quality of care without
nutritional supplements
4
5a
192
5.406
6,969
859,027
47
269
7,568
9,757
47
398
11.652
13.753
24
1,049.209;
368
10.733
12.770
26
617.724
195
5.483
1.785.971
7,069
47
597
17,508
20,664
24
552
16.127
19,188
26
1.164.833
Improved quality of CEmOC
Improved overall quality of care and coverage
with nutritional supplements
5b
602.646
Improved overall quality of care and coverage
without nutritional supplements
1.633.956
Source Auihors' calculations
22 I Wendy J. Graham, John Cairns, Sohinee Bliallacharya, et al.
I
I
I
:"F 001-032 ch26.qxd 05/24/2005
18:05
Page 23
Table 26.9 Sensitivity Analysis Results, South Asia and Sub-Saharan Africa
(incremental cost per DALY averted, US$)
i
Option number
Effectiveness
assumption
Met need
assumption
High
Low
High
Low
High
Low
148
142
240
255
144
113
100
180
193
104
163
163
326
311
164
147
143
241
373
144
150
144
242
260
149
213
142
240
446
152
109
143
240
204
136
203
153
250
203
210
227
189
92
83
77
151
86
70
64
61
114
66
104
90
85
166
94
91
83
77
228
86
93
84
78
151
89
191
83
77
326
123
84
83
77
130
82
84
66
93
84
87
123
79
Bost
estimate
Alternative compared with base package
South Asia
2
Increased primary-level coverage
3a
Improved overall quality of care with nutritional supplements
3b
Improved overall quality of care without nutritional supplements
4
Improved quality of CEmOC
5a
Improved overall quality of care and coverage with nutritional
supplements
5b
Improved overall quality of care and coverage without nutritional
supplements
Inpatient cost
assumption
Sub-Saharan Africa-
2
3a
3b
4
5a
5b
Increased primary-level coverage
Improved overall quality of care with nutritional supplements
Improved overall quality of care without nutritional supplements
Improved quality of CEmOC
Improved overall quality of care and coverage with nutritional
supplements
Improved overall quality of care and coverage without nutritional
supplements
Source: Authors’ calculations.
companion is with or without increased coverage (options 5a
and 3a, respectively). This difference reflects the high burden
from low birthweight in South Asia and, thus, the gain from
nutritional supplements.
Comparing the content of the three most cost-effective
intervention packages (3a, 5a, and 2) suggests that much can be
achieved through improvements at the primary care level
Improved quality in relation to managing complications-in
other words, the provision of BEmOC-and increases in cov-(
erage (a combination of options 3a and 2) at the primary level
taHe 26 7 Th 7 7”
ICERS 'ha” ,h°Se shown in
table 26.7. Tins finding Is consistent with the Commission on
Macroeconomics and Health's emphasis on close-to-client
services (CMH 2002), and it is highlighted further in chap
ter 53. As noted earlier, given the importance of prompt interventmn in the event of obstetric complications, the effective
ness of intervention packages that may reduce delays by bringmg services closer to communities is hardly surprising
. The benefits from option 2 were achieved essentially by
increasing prenatal care coverage from 50 to 70 percent
because our model assumes that those women taking advan. ge of professional delivery are those who have also had pre
natal contact. Prenatal care is, thus, a crucial entry point to the
health system. Small changes in prenatal care coverage (20 per
cent) lead to larger numbers of women also benefiting from the
rest of the care package in terms of obstetric first aid and
CEmOC.
This issue is important for safe motherhood and newborn
health, because the role of prenatal care has been subject
to intense debate about its benefits relative to resource use
r"d Van Lerbcrghc 2001; Maine and Rosenfield
1999). Much of this discussion has focused on the lack of evi
dence on the direct contribution of prenatal care to reducing
maternal mortality (McDonagh 1996; Rooney 1992), which in
turn, is explained partly by the poor performance of at-r’isk
screening tools. However, differentiating the contribution to
the preventmn of maternal deaths of the prenatal care compo
nent alone is difficult. Ultimately, life-saving interventions
depend on the functioning of the entire health system, including an effective referral network.
Our model also made assumptions about women's willing
ness and capacity to respond to referral to higher levels of care
n> case of complications. This willingness and capacity depend
on many factors and are undoubtedly also driven by commu
nities perceptions of quality of care. As noted earlier, coverage
rates of prenatal care are already high in many Sub-Saharan
Afncan countries, but significant socioeconomic differentials
are apparent within countries. Our model does not address this
Maternal and Perinatal Conditions I 23
I
'■'32 Ch26.qxd 05/24/2005
18:05
Page 24
equity dimension but, given the recent work showing higher
risks of maternal death among the poorest groups, targeting
disadvantaged women for improvements in uptake might
be worth considering (Gwatkin and Deveshwar-Bahl 2002;
De Brouwere and Van Lerberghe 2001).
Whereas option 2, increased primary-level coverage, relates
predominantly to the demand side of the health system
tWilliams 1987), the most cost-effective packages (3a and 5a)
locus on the supply side, particularly at the health center level.
I Ik- latter packages are particularly relevant to thc baby, includ
ing screening of the HIV status of the mother and treatment
wth antiretrovirals at the time of delivery to reduce the risk of
mother-to-child transmission, as well as provision of antimalanals. As a consequence, these options have a particularly
marked effect on the burden from perinatal conditions,
accounting for two-thirds to three-fourths of the total DALYs
averted (table 26.8). Note that these cost-effective options
include a doctor at the health center level to provide all six
Emoc functions. In some situations, highly skilled midwives
K.l be able to act in this capacity, which would reduce costs
and further increase cost-effectiveness.
The most comprehensive packages in our model provide for
improved quality of care and coverage at both the primary and
L“|
y V (Op"OnS 5a and 5bL Costing US$1.79 and
■ 1.63 per capita, respectively, in Sub-Saharan Africa (as
kulatod from the total costs of these packages shown in
■ lit 26.8 and divided by the base of I million people), these
■ CO also the most expensive packages. Not surprisingly, there
fore, these two opuons avert much higher numbers of DAI Ys
With the package that includes nutritional supplementation'
*
have identified even more cost-effective intervention packages,
such as a combination of options 3a and 2.
ECONOMIC BENEFITS OF INTERVENTION
A narrow definition of the economic benefits of safe mother
hood interventions would focus primarily on the impact of
maternal mortality and morbidity on household investment
and consumption. Investment in this context refers not so
much to financial investment as to investment in improving
housing conditions, agricultural productivity, education, and
so on. The key elements to capture include the loss of produc
tivity and the disruption of planned investment and consump
tion. In addition to the loss of a woman’s own productivity
consequent effects are likely on the productivity of other
household members-effects that may be particularly long
ived m the case of young children whose health and education
suffer because of their mother's death. Thc household will also
be worse oft because it will have diverted resources from pre
ferred consumption and investment activities in response to
the health crisis. Thus, recognizing the dynamic consequences
of maternal death and disability and selecting an appropriate
time horizon for the analysis are important.
1 he potential benefits to individual households arising from
investments in safe motherhood are relatively clear, although
Challenges m quantifying and valuing them remain. Thc bene
fits may, however, be more widely spread in that improvements
in safe motherhood may reduce poverty, which in turn may
* stimulate economic development. Increased economic develop
(fibleTsTGPa" ,lmeS 35 many DALYS 35 ,hc baSe packa8c
ment may then feed back into further improvements in materKes that 1 ",1
',SCnCrallylll'-’mos,ct>n>Prehensivepacknal health, generatmg a virtuous cycle. The mechanisms whereges-that is, those that result in the greatest gain in quality
bf
dranges m maternal health affect other parts of the economy
live ^nd"8,
’
COSt ,'’C n’0S'-',re ofel> '’<» cost effee
toe and yct
analysjs found othcrwisc
ay be Ken Hied by a close examination of the influence of
maternal health on productivity and educational attainment
Partly be explained by the linear assumptions about effective
number of links may exist between safe motherhood and
■ess in the model and the assumption that the marginal
,<f
;
--aot. Such a finding also stresses both^the impork performance ol the health care system; therefore, strategies
«> niprove safe motherhood may be a means of achieving
Kt o( a well-lunct.onmg health system (rather than an excesIder health serv.ee improvements (Goodburn and Campbell
1 focus on one element) and the absence of any quick fix
2001). lowed (2oo(), 213) n„,cs ,|ki1
J ,
Moreover, we did not model these more compr he s ve
' ’"ons as perfect but unrealistic scenarios. We also fl
allowed for 30 percent of pregnant women not attend g n
■■a ai care. 50 percent of severe complications at a
capaeny to respond to obstetric emergencies, it is necessary to
have the sk.lls and supplies to deal with trauma, give blood
theatS and.aneSthesia’ and
» functional operating
• hiis, initiatives in safe motherhood could be an entry
pomt for wider health sector reform and improvement.
her of assumptions for which data
are extremely limited, and it
remains fairly crude, having Seen sul,_ lu unly a 1Hn|tcd sen_
ibjcct to only a limited
sitivity analysis. Second,
many comparisons are possible from
™r model, but we have selected only six. Thus'
- - > we may not
LESSONS FOR IMPLEMENTATION
24
Join. Canns, Solunee
The fi„di„gs from thc C£A i|R|icatc
he reduced burden that may be achieved by implanting
selected packages of interventions. Such inmlenw,
Such implementation
e, nl
I
' ■'1 032 Ch26.qxd 05/24/2005
18:05
Page 25
assumes, first, that decision makers accept the evidence and are
willing and able to act and, second, that an enabling health sys
tem environment exists within which the requisite scale and
quality of care can be effectively delivered. These factors are not
peculiar to safe motherhood, but they undoubtedly help
explain the significant gap between evidence and action that
to hospitals and financial barriers to access on the part of the
poor.
fhe financing of prenatal and delivery care services at an
adequate and sustainable level is a subject of much debate
and uncertainty, given the difficulty of distinguishing these
elements from broader health expenditure categories (De
Brouwere and Van Lerberghe 2001). Given the low level of
overall per capita expenditure on health in developing
countries—estimated at US$13 in 2002 for the poorest 49
countries (Bale and others 2003)—attaining our base interven
tion package (costing approximately US$0.41 per capita in
South Asia and US$0.60 in Sub-Saharan Africa) does not
sound unrealistic at current resource levels (see table 26.8, and
divide by base population of 1 million people).
The effects of health sector reforms, particularly decentralizat.on of management and budget holding, appear to be mixed
in terms of increasing resource flows into maternity services,
with both apparent positive benefits, as in Bolivia (De Brouwere
and Van Lerberghe 2001), and negative effects through
the exacerbation of inequities (Russell and Gilson 1997)
Effective management decisions on finance, access, and quality
require information, an essential ingredient for stimulating
action. Io allocate scarce resources where they are likely to
^h.eve the greatest gain, countries need information to assess
the burden of ill health, evaluate the performance of current
many argue is one of the main obstacles to progress (Godlee
and others 2004; Villar and others 2001). The gains from bridg
ing this gap would be significant: the MDGs for child survival
and maternal health might become more than mere rhetoric
lor poor regions if intervention packages of the scope and
nature described here were implemented. The most costeffective of the packages averted nearly 50 percent more direct
maternal deaths than the base package. This gain would be
encouraging, but the prospects for achieving it by 2015 are
weak (Johansson and Stewart 2002).
At the macro level, a supportive policy environment clearly
is crucial, as noted earlier. At the micro level, an enabling health
system implies a reduction in the disequilibrium between the
demand and supply sides (Williams 1987), with particular
attention to three interrelated issues: access, quality, and
finance The CEA reported in this chapter emphasizes the
potenoal benefits to mother and baby of improved access to
care, particularly the importance of entry to the health system
through primary-level services. The increases in coverage could
e achieved by a variety of mechanisms but clearly require both
intervention
scope
for improvement
and
demand- and supply-side interventions.
.
, strategies, identify
<the
--------------ana
On the supply side, this chapter has shown that improved cost Zf
8n
loo-rP -i
bX eval^ng viituts
effects and
and
cc
alltv nfr,r.
nprovea
cost-effectiveness
MrCarthvy«and Ross 2ooi).
quality
of care at
at u_n.
both ..
the primary
primary and
and secondary
levels
c“venrs.(Lawn.
(Lawn: McCarth
both
the
^e"2h°U?hJhe challcn8es that the poorest countries face
encompassing technical, infrastructural, and human resource
today
/ clearly
" 7" differ
C- in
■-1 many respects from those that developed
d.menstons (Ptttrof, Campbell, and Filippi 2002) is a particu
or transition
t
countries experienced in the past, six historical
larly cost-effective option. The widespread call for all women to
lessons provide
particularly
relevant lnSlghts
insight; First> exan
_ r
„,UVUUII1y rclcvant
ehver with skilled attendance immediately raises major ques71J? OfSUpP<)rt,VCpol,cyco,ltcxtsa,Hl individual dianipions
■ons about quality of care and capacity, because much of thp
—
me
of progi
jress in addressing maternal and newborn health, such
developing world faces an acute shortage, as well as an
unequal
as ose reported by De Brouwere and Van Lerberghe (2001).
geographic distribution, of health professionals
Second, historical data on the uptake of prenatal care demonOur CEA assumes that redistributing human resources
'S.thm countries will accommodate the increased uptake of' strate that ccom mu mt y- based providers and advocates played a
crucial role. Third, t.ie
tin role of various professionals and profescare by women, although the most effective mechanisms for
sional bodies has inot always been positive, particularly as
achieving th,s goal, such as incentives, use of nonphysicians
regards the “war" betw<
and n,creased private sector involvement, have not yet been
■^ecn advocates for home and institutional
established (De Brouwere and Van Lerberghe 2001), What is
fdeliveries
. . . (Koblinsk
,
y and (:a"'Pl»ell 2003). Moreover, good hisoncaJ evdence tndicates that excessive rates of forceps deliver
clear, however, is the importance of the interplay between supply and demand, with the
mater I
1,.terVenti0ns were si8nifi«nt contributors to
supply of quality care stimulating
1 a erual mortality m countries such as the United Kingdom
demand for care and vice versa,
i- Quality care includes an effecand the United States (Buekens 20(H). Fourth, primary level
live referral system (Murray and others 2001)
to ensure the
depends on an effective referral system being in place to
requtred match between the various levels of care different
and Va “l 'l
^bieS
differCn' ,imeS (De Bro“™re
and %U Lerberghe 2001). Such systems require not only
to
/feT"/0 7PP°r‘ tra'’SBOrtalio'’. cotrnnunicattons, and feedback mechanisms, but also structured fee and
exemption strategies to reduce both inappropriate self-referral
). Iflh to reduce the burden of maternal and perinatal
access foAhe" S,,S‘em
b'3'*
finanCing nlUS' facilitate
ccess for the poorest groups and guarantee service quality
'"f
Lcrber8he 2001)- Anally, the role of pop
tilation-based information on births and maternal deaths was
Maternal and Perinatal Conditions I 25
I
001-032 ch26.qxd 05/24/2005 18:05 Page 26
crucial m ensuring that actions were locally relevant (Sorenson
and others 1998), in demonstrating progress, and thus in stim
ulating further action. This crucial role is particularly apparent
in the literature on several European countries in the past cen
tury (Graham 2002; De Brouwcrc and Van Lcrberghc 2001).
change, such as a particular increase in the uptake of prena
tal care, may not be known. Thus, the ICERs may be too low,
in that they do not fully capture the costs of the intervention.
Estimating cost-effectiveness. More sophisticated economic
models need to be developed to facilitate the evaluation of a
wider range of safe motherhood strategies, particularly as
better primary evidence becomes available from other stud
ies and initiatives using a variety of outcome measures
(Cairns, McNamee, and Hernandez 2003). Similarly, proba
bilistic sensitivity analysis would be a valuable development
that would permit fuller exploration of the uncertainties
regarding the model’s parameters.
RESEARCH AND DEVELOPMENT NEEDS
I he priorities for research and development arising from this
chapter need to be put in the context of wider requirements for
safe motherhood and newborn health that have been well articU ate- L sewhere (see, for example, Bale and others 2003) The
general heading under which the specific needs emerging from
ns chapter can begrouped is evidence-based decision making,
'hich has five crucial requirements:
CONCLUSIONS
Ing scarce health care resources in poor counties'0 a110"''
maternal and Perinalal conditions represented the sin-
making efforts to improve the scope and quality of data on
on
thc burden from maternal and perinatal conditions
■ carrying out robust evaluation of the costs and effectiveness
of intervention strategies
fo'/'8"' “ntr'bUtOri,° thc 8lobal burden °f disease, at nearR. 1 *WCC"t “ l0,al DALYs (Mathers and others 2004).
nat on J a d3'.
Z'7 Sta'Cd
3 Pri°rity at both
J
nd ,nternahonal levels, but the track record of trans-
’
■
.. .......................................................... di[^.mdUn!
assessment of their performance.
Wetl and Fernandez 1999). Many reasons account for the lim-
Within those major areas, specific topics relevant to the CEA
undertaken here include the following:
cr
":"g ",e bujrde" °fpcrimual conditions
reater clarity and consensus are needed on the scope of this
important burden category and the implications of signifi
cant current exclusions, such as indirect maternal conditions
n stillbirths. Practical assessment tools are needed to
enable meta-analysis and other modeling approaches to svs
^-^--^-onstra.nts.^Xp^
edge ex st with regard to the levels and consequences of
™>erna| morbidity (Say, Pattinson, and Gulmezogfo 2004)
■he contribution of iatrogenic factors, the unpredictability of
n.a ernal complications, and the levels of mortality. Most
_
“7 B‘'PS rCqUlrC si8nifican’ developments in relation to
vat ab e measurement tools and in poor countries' ca ad
use them as part of routine health surveillance. These
1 nprovements not only are needed to inform future CEA bu
also have w.der implications for global health monitoring
IPanent.ngcl.angc.ln addition to evidence on the content
» mtervent,on strategies, assessments of how to impleme
changes are urgently needed. A limitation of our anZs i
■ e progress, especially in the poorest regions of the world, and
researchers offer many interpretations of the bottlenecks. Lack
o evidence on the size of the burden and on the effectiveness
o alternative intervention strategies figures prominently in
these interpretations.
z
The modeling in this chapter is, therefore, based on imper
fect knowledge and needs to be supplemented with data from
QZe
.“‘ions- T'’e flndi"8s d°. however, provide some
sent th 7 t "’t0 Progran’matic “Phons that may repre
sent the optimal use of resources in South Asia and Sub-
F rstTr
'n ‘hiS C°nteX‘'three iSSUCS dcSCrW cn’Phasis.
rst for intervention packages to achieve the degree of costffe -veness shown here, improvements are needed across
health systems and both the supply and demand sides need to
be addressed Second, crucial entry points t() this sys,t.1„ Z, “
Hcvcd at the primary level, particularly through prenatal
care. The effect of increasing the volume oLonte, n « “
with these services is likely to manifest itself in an inc“
Pioportion ol deliveries with skilled attendance and of deliveries
>n which women obtain access to emergency obstetric care
Final y the quality of these services is crucial, and even with
y
percent uptake of care, benefits can still be achieved in
ha>. even though [he mo(|cl niay be * reasonab|e
■a ion of the resource and health consequences of different
■nterventrnn packages, the way to achieve the required
Initiatives to improve the quality of care, particularly at a
pnmary level, thus appear to be cost-effectZoptions for the
26 ' Wendy J Graham. John Cairns. Sohmea Bhattacharya. e( al.
I
i' qx'l
O'., 2-1 /200r,
IRtD'",
<b
Paqo 27
poorest regions of the world. Overall those findings appear to
I'-'hI support to a sale motheihood and newborn health
strategy that is close to the client and boosts community confi
either maternal or perinatal complications. About 2 percent of
mothers are assumed to require treatment lor preterin delivei y,
and 1 percent for premature rupture of membranes.
dence in health systems.
In pi actice, the proportion ol women with serious coinplica lions receiving comprehensive emergency obstetric care varies
widely, from 3 percent in Cameroon to 75 percent in Sri I anka
ANNEX 26.A: CEA MODEL ASSUMPTIONS
(Averting Maternal Death and Disability Working Croup on
We assumed that there are four primary-level health facilities
'health centers) and one secondary-level care facility (district
hospital I for every 5()0.0()() people. We estimated the numbers
of pregnancies and births from the crude birth rate for each
region. We assumed that pregnant mothers attending for rou
tine prenatal care are equally distributed between the live facil
ities and that each facility provides similar routine prenatal and
delivery care. Routine prenatal care is assumed to comprise
four visits—except for mothers with complications, who make
six visits. Mothers with complications are referred to the dis
trict hospital after their first visit if (hey cannot be treated at the
health tenter. We assumed that complications such as anemia
Indicators 2003). The scenarios considered in this chapter
assume that cither 20 or 50 percent of women with serious com
plications reach secondary care, and that 50,70,80, or 90 percent
of those women receive the elements of comprehensive emer
gency obstetric care that they need, depending on which inter
vention package is being considered. For the sensitivity analysis,
we used low values of 30,50,60, and 70 percent and high values
of 70, 80, 90, and 95 percent. We assumed that ambulances arc
available, so that when the proportion of mothers with severe
complications reaching secondary care is increased, the addi
tional costs are only the additional driver time and the increased
costs ol running and maintaining the vehicle.
The prevalence and incidence of different maternal condi
and sexually transmitted diseases are treated without referral to
secondary care, as are preeclampsia and incomplete abortion, if
a doctor ,s present at the facility. The average number of bed
days is assumed to be three clays for normal deliveries and six
days for cesarean section and other complications. Table 26. AI
shows the U.S. dollar costs per inpatient bed day used in the
tions are taken from the WHO mother-baby package (WHO
1994). World Health Organization estimates of the burden of
dillcrent maternal and perinatal conditions (WHO 2()()ld)
have been applied to a population of I million, with a particu
lar crude birth rate to generate an estimate of the potential
number of deaths that could be avoided, the years of life that
main analysis and in the sensitivity analysis.
We assumed the existence of excess capacity, so that an
increase m prenatal care coverage from 50 to 70 percent would
"ot reqtnre an increase in the number or capacity of existing
health care fac.hties or in the number of personnel, and tht
increased costs would mostly be increases in variable costs For
mcreased coverage of prenatal care, we assumed a need for
mcreased expenditure on education, information, and commmmam.n. Enhanced prcnatalcareandct.mprehcnsiveemer-
could be saved, and the DALYs that could be averted The
assumptions regarding the effectiveness of the interventions
with respect to maternal and perinatal conditions were based
prinrlrifv
‘
/ on the WI lO’s mother-baby package and a review of
the hleraturc; they are shown in table 26.A2. We assumed that
each intervention has the same effect on the number of deaths
years ol hie saved, and DALYs. The effectiveness of interven
tions is assumed to be additive.
>t- n. y obstetric care ate asMuned to ,e,|uire additional expend!
lines on ha,mug, assumed to be It) percent of total personnel
costs, hr assumed that the additional costs of basic emergency
ACKNOWLEDGMENTS
obstetric care compared with obstetric first aid arc largely due
I” provtdmg doctors at each health center. We also assumed
that 8 percent of toothers require cesarean section as a result of
We would like to thank the many individuals who helped P,e
l-me this chapter. In particular, we acknowledge the expert
'"I"" yarding perinatal conditions Iron, loy l awn ..... I |el|.-..,
m «0S,S Per l"Pa,ient Bed Day' S°U,h Asia a"d Sub-Saharan Africa
South Asia
Cost of inpatient bed day
Best estimate
lo.v
Secondary level
6.51
8.50
2.64
High
,•
Sub-Saharan Africa
Primary level
3 45
14.52
18.94
c^ir'ilntions
Primary level
6.17
1.92
41.79
Secondary level
8.05
2 51
54 52
M.ileuiiil ■iikI Pcritiaial I jinilihnn.'; I 27
*
A
■'•’.-9’2 Ch26.qxd 05/24/2005
18:05
'I^
Page 28
Table 26.A2 Assumed Effectiveness of Interventions
(number of DALYs averted)
analyses; and level C is assigned Io ease series, ease studies, „r expert
opinion.
’'
I- ■"’•y l'apter does not deal with tertiary and specialist levels of care
or with rehabilitative care or care for chronic conditions
5. The six functionsestimate
of BEmOC
are (a)
administering antibiotics intraLow
High
Vl'’‘’«'sly<’r intranu1scularly1(b)adnHnisIering<>xvt<Kicsintiaven<H,sh ...
"> ■•"omu nl.nly. (< ) manually removing the placenta, (d) adminhie,inaimconvulsants mtravcnously or intramuscul.uly, (e) curving out inM.u’
menta Ide hvciy.and (f) removing retained productsol conception I he two
^dtlnmal nttetions in <Xln0C
|>|(,„d ,ram,llsi(am| tc;OTi
Best
Condition
Malein^l
Hemorrhage
85
75
76
Sepsis
Hypertensive disorders of pregnancy
(including eclampsia)
Obstructed labor
80
Unsafe abortion
75
80
90
70
90
71
95
75
70
95
90
<nt. l or a factltty to |,e regarded as a BE„,()C or < T.tnCHi site, respeetivel,
I n>us perforn, all stx or all eight functions regularly and must I,' assessed
every three to six months (UNEPA 2003).
Perinatal
REFERENCES
Low birthweight
In context without nutritional supplements"
In context with nutritional supplements"
8
3
14
28
23
44
40
35
60
90
80
60
Birth asphyxia (including birth trauma)
In context without enhanced delivery care package"
fit context with enhanced delivery care package
infections. including tetanus
Sepsis (newborn)
70
65
60
55
40
35
60
55
HIV/AIDS
AbouZihr.C. 1998. •‘Maternal Mortality Overview." In Health I >i,nenm",<
o) Se.x am Re^htction. cd. C. |. Murray and A. I). I ope/, I 11 6.|
Geneva: World I lealth Organization.
—IW.“Di.sabi|ily.Adjuslcd Life Years and Keprodueiive llealdr- A
Critical Analysis. Rcprmlnctive Ilealth Matters 7 (I D: I |;<
Kuird,
Abou/ahr.
,
and I. Wardlaw. 2001. •'Mau , „.,l . ...... .
7'HO) so,«.S!OU oo-
80
Averting Ma.ernal Dead, and Disability Working <irtn,,
200:. 1 rograni Note: Using UN I'rneess Indicators h Assess Needs in
Innergency Obstetric .Services: M.rrocer,, Nicara,..,., and sIri I bite:milmml /ounml 0/ Gynncail^y
obslcttits BO; 222-30 '
...
Bailey. !>.. I. Szas/di, and H. Sebiebcr. !9»5. “Analysis of ,|,c Vi, d I vents
O-p.rt,ng Systen,
,hc ............. ..
K n s
Zupan, rlutnks arc a|so givcn
Qur col|cagues a(
Un,versify o Aberdeen, particularly Joyce Boor, Julia Hussein
•nuna 'khrt.rtl,, Nara Tagiyc.va.Mihlc, .|lu| K;lrc|1 wjUc||
;
eknowledge and thank onr colleagues Paul McNamee anti
P " —audeZ from .he Heald. J,.......
l;cseJ
nXXS
.. .... .... Wi'rki"s
(•■ National Academy of Sciences and Institute ol Medicine.
'‘- II. L. S I.. Cutis, ami S. Alayon. ?(»(»). ” |I(.I1(|S
Un.t at the Utuversny of Aberdeen for their thorpugh review of
*he cost-effeettveness analysis. Thanks also to orn exper ' " e
-nrbers: Heanna Ashley, Gary „arnisla(It,
”
'updletsc , Jdly Jreland, Joy I.awn. (,dl K|ufio.
■ Nyncux■ Ashalata She,ty.SribalaSripatl, Vijay Ku,nar Tandl
Suntesh 1 ho,nas, ami Jelka Zupan.
'"U'«tnes
|)(.|ivc,v (• |ti. •
si
Department ol Homeland Security Analytical Studies' 7
Bell J., I Hussein, B. Jcntsch, G. Scotland, (:. Bullom-h md W I c
2003."ImprovingSkilled Attendanceal Deliv , A P a' •
"I the BAN Strategy Developnient li,„|“ B,„h w '„
i
...
NOTES
an
^''''•'''^'^(.ynaaloguaSutmhnavi,.!
Jha"*’cs ",c Probability (,f
,,n''^;|l“BM.,rehal.andVDe|1,.,1„,.v,e.?;:::;
•loldhntl,: le. Us tin beyond the RheUnics"I- Skilled Ath iiil.im c at
rn>i>iml Meilume ami
International Health 9 (6): 653-54.
2- Afghanistan, z\ngola. Bangladesh ( him ih. i
.
2)of ( ongn, I'thiopii Imli i in I
’ lc ^tlllocr«itic Republic
and ilgt.d'. .......
'
Ke">'a'
IlaLnia,
-V We use a <
si''B/
’l'll>'« waydiSli,Kli,>,,r.,rlevels,,lcvidaKe level,A
refers to evidence frot
of trials; level R relate;
„M.ra,ulnm,zed studies, often wil|, „ndlivi,ri.lte
(.aims, J., |> McNamee, and R. Hernandez 2003 “M
( alder, A. A., and W. Dunlop, eds. 1992,
High Risk Pregnancy. Oxford,
U.K.: Butterworth-Heinemann.
28 '
I
I
I
l-Ol.? ch26.qxd 0S/24/2005 18 : OS Page 29
4
Carroh, G., C. Rooney, and J. Villar. 2001 .“How Effective Is Antenatal Care
in Preventing Maternal Mortality and Serious Morbidity? An Overview
of the Evidence." Pucdiatric and Perinatal Epidemiology 15 (Suppl. 1):
Gwatkin, D., and G. Deveshwar-Bahl. 2002. “Socioeconomic Inequalities
in Use of Safe Motherhood Services in Developing Countries." Paper
presented at the Inter-Agency Safe Motherhood Meeting, London,
February 6.
Chamberlain. G„ ed. 1995. Turnbull's Obstetrics. 2nd ed. London:
Hall, M., S. MacIntyre, and M. Porter. 1985. Antenatal Care Assessed.
Aberdeen, Scotland: Aberdeen University Press.
Churchill Livingstone.
Cleland, J., and M. Ali. 2004. “Reproductive Consequences of
Contraceptive Failure in 19 developing Countries." Ol>s(crrics and
Gynecology 104 (2): 314-20.
Hoj, 1... D. da Silva, K. I ledegaard, A. Sandstrom, and P. Aabv 2001
“Maternal Mortality: Only 42 Days?” British Journal of Obstetrics and
Gynaecology 110 (11): 995-1000.
CMH (Commission on Macroeconomics and 1 lealth). 2002. Improving the
Health Outcomes of the Poor. Report of Working Group 5 of the
.ommr.-.ion on Macroeconomics and Health. Geneva: World Health
< Irgam/ation.
Hussain, R„ F. E Fikree, and IL W. Berendes. 2000. “The Role of Son
Preference in Reproductive Behaviour in Pakistan." Bulletin of the
World Health Organization 78 (3): 379-88.
1 IU,“'’eSS)'' C? R-MT. Tan-Torres Edejer, and D. Evans. 2003.
Generalised Cost-Effectiveness Analysis: An Aid to Decision Making
in Health. In Making Choices in Health: WHO Guide to CostEffectiveness Analysis, ed. T. Tan-Torres Edejer, R. M. Baltusscn, T.
Achm IL Hutubessy, A. Acharya, D. B. Evan, and C. J. L. Murray,
277-88. Geneva: World Health Organization.
Creesc, A., andID. Parker. 1994. Cost Analysis in Primary Care: A Training
Manual for Programme Managers. Geneva: World Health
Organization.
Dayaratna V.,W. Winfrey,W. McGrcevey, K. I lardee, K. Smith, E. Mumford
and others. 2000. Reproductive Health Interventions: Which Ones Work
and What Do They CostfWashington, DC: Policy Project.
De Brouwere, V. and W. Van Lerberghe, eds. 2001. Safe Motherhood
Strategies: A Review of the Evidence. Vol. 17 of Studies in Health
Services Organisations and Policy. Antwerp Belgium: ITG Press.
Donnay, E 2000_“Maternal Survival in Developing Countries: What Has
Been Done; What Can Be Achieved in the Next Decade.” International
Journal of Gynecology and Obstetrics 70 (I): 89-97.
Eclampsia Trial Collaborative Group. 1995. “Which Anticonvulsant for
omen wuh Eclampsia? Evidence from the Collaborative Eclampsia
Inal. Lancet M5 (8963): 1455-63.
P
^“m
n B‘ ^diO’ and S’ TraOre’ ,9"- “Assessment of Maternal
Mortality and Late Maternal Mortality among a Cohort of Pregnant
i oo'hT ^5lako’Mali”British ,ournaI Ofobs,etrics and Gynaecology
Jamison, D. T, J. S. Jamison, J. Lawn, S. Shahid-Salles, and J. Zupan. 2004
Incorporating Deaths Near the Time of Birth into Estimates of the
Global Burden of Disease." Working Paper 26, Disease Control
Priorities Project, Bethesda, MD.
Johansson. C„ and D. Slewart. 2002. "The Millennium Development
Goals: Commitments and Prospects." Working Paper I, Human
Developmen Report Office Working Papers and Notes, United
Nations Development Programme, New York.
Jowctt. M. 2000. “Safe Motherhood Interventions in Low-Income
Countries: An Economic Justification and Evidence of Cost
Effectiveness.’ Health Policy 53 (3): 201-28.
Khanum, P. A., M A. Quaiyum, A. Islam, and S. Ahmed. 2000
Complication of Pregnancy and Childbirth: Knowledge and Practices
CenTefoTn" h IB’ngladcsh” Workin8 PaPcr '31. International
Centre for Diarrhoeal Disease Research, Dhaka.
Evans, T, and S. Stansfield. 2223.
2003. “Health Information in the New
Millennium: A Gathering Storm?”
Bulletin of the World Health
Organization 81 (12): 856.
A'’ andr°' Canipbell> eds- 2003- Reducing Maternal
Zd^a
lo7
,7 rOn2
O,:Vi(,, DC: World H0nd,,W
Jamaica, and
Zimbabwe.
Washington,
Bank. ‘
Forgo, W. 2(X)|. “Managing Newborn Health in the Global Cm
innninity.”
American Journal of Public Health 91 (10)- 1563-64
^Zms- F 200,A“fng Huma"
in Maternal Mortality
Koenig, M. A V Fauveau, and B. Wojtyniak. 1991. “Mortality Reductions
from HeaIth Interventions: The Case of Immunization in Bangladesh "
Population and Development Review 17 (1): 87-104.
Cvn Z?
,O S,ra,c8y" ‘"“■'rnational Journal of
Gynecology and Obstetrics 75 (1); 51 -60.
1
Ga>; J K. Hardee. N. Judice, K. Agarwal, K. Flemming, A. Hairaldn
and Others. 2003. Wru, iVo*.. A poUcy and Prog^
Evrderrce on F,,„,,ly Pl„„„i„g, Safc M„lhcrhmd Jltd STI/HIV/A DS
Ou^rnrrns. Safe Motherhood Module I. Washington, DC: X
Kurjak, A., and I. Bekavac. 2001. “Perinatal Problems in Developing
Countr.es: lessons Learned and Future Challenges." Jou °«l f
Challenges.” Journal of
Perinatal Medicine 29 (3): 179-87.
6
/uurnai oj
KUS",I'<’'1T ’ Rons"’a"sI • Van der Paul. 2000. "Perinatal Mr.mliiv
Annbutahle to Complications of Childbirth in Matlab, Bangladesh"
Bulla,,, of the World Health Orgmtio,, 78 (5): 62|_27. 8
Ce Go N
“.PoPula'ion »nd Reproductive Health: Where Do We
Go Next? Anwncw lo„r„„l of Public Heal,I, 90 (12)- 1845-47
Lawn |. E„ B. ) McCarthy, and S. R. Ross. 2001. The Healthy Newborn- A
Dita e1'Com"''1 ,Pr"sr‘"" Ml""Vrs- AUanta. GA: Centers for
ease Control and Prevention and (’ARP Pit.-. //
gov/reproductiveheahh/health.newbornhtnK
h,tp://—de.
Loudon, I. 1997. “Midwives and the Quality of Maternal Care” In
Mtdwtves Society and Childbirth: Debates and Controvert in tZ
.
British Medical Journal 322 (7291): 917-20
7
Cy‘
Graham W. |. IW8. "Every I'regnamy Faces Risks."
Pltmnetl
Parcnihootl
Challenges I: 13-14.
for McMurins Maic™'
'^“Hmihd Lh E' R'Z"Tr!CC’ '■ S- M|. “"<1 )• A. Cairns. 2004 "The
363 (9402k 23T
8 Ma"r"al DCal1' ■1"d
‘"tToikghA11 ' HUSS'in- 2°04- “The
'» Count.” Lance, 363
'
-d N« I^mlcd^rla,,d 3nd A-
Ra,fCr,y’ l8Q-200- L-d-
Maine, I >., and A. Roscnlield. 1999. "The Safe Moth.
icrhood Initiative: Why
as t Stalled?” American Journal of Public Health 89 (4): 480-82
'
Marston, C., and J. Cleland. 2003. “Do Unintended Pregnancies Carried to
erm Lead to Adverse Outcomes for Mother and Child? An Assessment
m Five Developing Countries.” Population Studies 57 (1 )• 77-94 '
... -......... ..
Maternal and Perinatal Conditions I 29
I
: ' P 0011- 0 32_ch26 . qxd 05/24/2005
18:05
Page 30
McCarthy, J„ and D. Maine. 1992. “A Framework for Analyzing the
Determinants of Maternal Mortality." Studies in Family Planning
Sadana, R. 2001. "(Juantifyinp, Reproductive Health and Illness.” Ph.I),
dissertation, I larvard School of Public I Icalth, Boston, MA.
N1C?<°nMr M’ l996-“ls An'ena,a> Care Effective in Reducing Maternal
Morbidity and Mortality?” Health Policy and Planning 11 (1): 1-15.
Save the Children. 2001. State of the World’s Newborns: Saving Newborn
Lives. Washington, DC: Save the Children.
Nl,llk? I”
Sco'land’ N- Tagiyeva-Milne, and J. Hussein. 2004. “Safe
Motherhood Program Evaluation: Theory and Practice.” Journal of
Midwifery and Women’s Health 49 (4): 338-44.
Mun)anja S R, G. Lindmark, and L. Nystrom. 1996. "Randomised
Controlled Trial of„a Reduced-Visits Programme of Antenatal Care in
Harare, Zimbabwe. Lancet (North American ed.) 348 (9024): 364-69.
Murray. C. J. L„ and A. D. Lopez, eds. 1998. Health Dimensions of Sex and
Say, L, R. Pattinson, and M. Gulmezoglu. 2004.“WHO Systematic Review
of Maternal Morbidity and Mortality: The Prevalence of Severe
Acute Maternal Morbidity (Near Miss)." Reproductive Health 1: 3.
http://www.reproductive-health-journal.eom/content/l/l/3.
prK
Mt" Dur(ie"
"o oL
Cambridge, MA: Harvard University Press.
Mu"ay’ S- E’ S- Davies- P- Kumwenda, and A. Yusuf. 2001. “Tools for
,E^C,ivcncss of District Maternity Referral Systems.”
Health I obey and Planning 16 (4): 353-61.
Mwageni E., A. Ankomah, and R. Powell. 2001. “Sex Preference
and Contraceptive Behaviour among Men in Mbeya Region,
770^
ta™ °fFarmly P,anm"X and Reproductive Health Care
i-i (Z). o>-o>.
NU7n pa,LE- n d B’ A"IOO,i|-K?8una- 1999. “Predictors of 1 lome Deliveries
m Raku District, Uganda. African Journal of Reproductive Health 3(2):
Pa,,I,"S0V" J2^avmg Mothers: Second Report on Confidential Enquiries
of HMkh™
‘
*999~2001’Pretoria: Department
Shehu, D. A. T. Ikeh, and M. J. Kuna. 1997. “Mobilizing Transport for
Obstetric Emergencies in Northwestern Nigeria.” International Journal
of Gynecology and Obstetrics 59 (2): 173-80.
Sheldon, T. A. 1996. “Problems of Using Modeling in the Economic
Evaluation of Health Care." Health Economics 5: 1-11.
Sorensen. G;, K. Emmons, H. K. Hunt, and D. Johnston. 1998.
Implications of the Results of Community Intervention Trials.”
Annual Review of Public Health 19: 379-416.
Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Mendez. 2001 “The
Burden of Malaria in Pregnancy in Malaria-Endemic Area.” American
Journal of Tropical Medicine and Hygiene 64 (1.2 Suppl.): 28-35.
Stoll, B. J., and A. R. Measham. 2001. "Children Can’t Wait: Improving the
729-33f°r ,hC W°r,dS P°OreSt InfantS" j0UrnaI °f ^diatrics 139 (5):
Fhonneau, P., N. Goyaux, S. Goufodji, and J. Sundby. 2002. “Abortion and
Odvria«?l0J,alhy in AfriCa” NCW
Journal of Medicine 347
1704—O->.
Tomkins A. 2001 “Nutrition and Maternal Morbidity and Mortality.”
lintish Journal of Nutrition 85 (2): 93-99.
’ ^C^’a0'
and V’ FiliPPi- 2OO2.“What Is Quality in Maternity
Care An International Perspective.” Acta Obstetrica et Gynecoloeica
UN (United Nations). 2002. UN PopMon Division Populnsion Esiimoics
Scandmavica 81 (4): 277-83.
and Projections, 2000 rev.
^'Families TndC San,arC.H.i- 2(J?3- “Empowerment of Women, Men,
UNUnA/ !U^daNriOnS PopU,ation Fund>- 20°3. Maternal Mortality
n N .
Commumries: True Partners for Improving Maternal
Update 2002: A Focus on Emergency Obstetric Care. New York: UNEPA.
and Newborn Health.” British Medical Bulletin 67 (1): 59-72.
unJCEF (United Nations Children’s Fund). 1999. “World Summit for
Pra^fNME ? u8’ u Walsh, 3nd M- Pol,s- 2004- “Sc“ing Priorities for
Children Goals: End of Decade Indicators for Monitoring Progress”
Safe Motherhood Interventions in Resource Scarce Settings” Paper
Executive Directive EXD/1999-03, New York.
6
Ju
P°pula,ion ^iation of America, 2004 Annual
Apr'!
°f Pub'iC Hca,,h’ Universi‘y of California-Berkeley, » Van Lcrberghe, W., and V. De Brouwere. 2001.
v,||ar J., H Ba’aqeel, G Piaggio, P. Lumbiganon. J. M. Bclizan, U. Farnot,
Prendiville. W. j., D. Elbourne, and 1. Chalmers. 1988. “The Effects of
and others. 2001. “WHO Antenatal Care Randomized Trial for the
outme Oxytocics Administration in the Management of the Third
Bruhh , /F:mt°VCrViCW °f ‘he Evide'1Ce fr°,n ^"‘rolled Trials ”
Bntish Journal of Obstetrics and Gynaecology 95 (1): 3-16
\ Villar. J„ and P^Bergsjo. 1997. "Scientific Basis for the Content of Routine
At.tcnatal Care 1. Acln Obslclrica el Gynecologic^ Sramlinnvim 76(1):
/'d' 2T Me"
PMnCr‘ '"^proiluaivc
iiuilth. Moving from Rhetoric to Reality New Delhi- P„n.,i ,•
Council South and East Asia Regional Office
P a"0"
Viliar I. M. Meriaidi, A. M. Gulmezoglu. E. Abalos, G. Carroli, R. Kulier
"‘’'AnZ N' R' C l99-’- " ............
.. ...... .
London:
and M. de Ont. 2002. Nutritional Interventions during Pregnancy for
the Prevention or Treatment of Maternal Morbidity and Preterm
l<O^ Rd LSt,a±’eC,r',annC On'ba? I999' ‘ C—doping Community.
Due to Um? At
7 a"rnal Morbidity
Mwbidi'y and Mortality
E Abortion: Pre-intervention Report.” Fast African
Medical Journal 76 (11 Suppl.): SI-71.
P
African
Rooney. C. 1992
(1,S.
Core n,,./
Hvw
WHO/MSM/92.4. Geneva: wfrld Health
Ifoose. D. I. 2003 "Potential Cost-Effectiveness of Nutrition Interventions
l'/CZd'erSC p,cBt>=ncy Outcomes in the Developing World"
A’urmt/ofNwrmon 133 (Suppl.): IM0S-I4S
P 8
tZZ ■NU'r,i,iOn and Ma'Cr"al
i"
Developing
"orld. Amer,can /r,„m,l ofCli„ical Nurririo,, 72 (Suppl.); 212-40
Russell. S.. and L Gilson. 1997. "User Fee Policies to Promote Health
l.'„r'ml hXi.i'ZkZtuT359?79heeP ’ Clo,l'in8" '"'"'"’"‘’"‘•I
30
o
“Z. X ^r^S"do",izcd c<""r"lkJ rrials"
ZZul
n”'1”,"' i"Kl
Gcr,lcr' ITO3' "Ma""’al and
Perinatal I lealth. In D.sease Control Priorities in Developing Countries
Oxford UK°O
!T> i-' A' MCaSham' a"d '■ Bobadil'a’ 363-^
uxlord, U.K.. Oxford Publications.
Wcil- ^’ ’nd ’l. Fernandez. 1999. “Is Safe Motherhood
an Orphan
Initiative? Lancet 354 (9182): 940-43.
W“si,K',u lr" Ki"Z’ S- K- Khalry' S- C- LeCler<li E- K- Pradhan S R
Lo v n”' ’He r l999 "Double "'"'d. Cluster Randomised Trial of
Low Dose Supplementation with Vitamin A or Ben Cnminnn
XX)^0
in Nepaf ABrZBZS'Z
Wlio (World Health Organization). 1986. "Maternal Mortality Helping
Wotnen off the Road to Death." Woriri Healll, OrgonieoUof^
//end/ J Gfahain. John Cairns. Soln
unco Bhattacliarya, et a)
I
FCF 001 032 Ch26.qxd 05/24/2005
18 : 05
Page 31
.1992a. International Classification of Diseases and Related Health
Problems, 10th rev. Geneva: WHO.
. 1992b. The Prevention and Management of Unsafe Abortion.
W HO/MSM/92.5. Report of a Technical Working Group. Geneva:
WHO.
--------- . 1994. Mother-Baby Package. WHO/FHE/MSM/94.11. Geneva:
WHO.
1999. Mother-Baby
Package
HO/FCH/RHR/99.17. Geneva: WHO.
Costing
. 2004c. Unsafe Abortion: Global and Regional Estimates of the
Incidence of Unsafe Abortion and Associated Mortality in 2000. 4th ed
Geneva: WHO.
.2004d. Global Burden of Disease for the Year 2001 by World Bank
Region, for Use in Disease Control Priorities in Developing Countries,
2nd ed. Bethesda, MD: National Institutes of Health
http://www.fic.nih.gov/dcpp/ghd.htinl.
Spreadsheet.
WHO-CHOICE (Choosing Interventions That Are Cost Effective). 2004
"Choosing Interventions That Are Cost Effective.” Geneva, http://
. 2002. Reducing Risks, Promoting Healthy Life: The World Health
Report 2002. Geneva: WHO.
oiglZhWh° ■il”/whoSis/nienU-cfm?pa‘h=whosis’cca&languagc:=
——^2003. Making Pregnancy Safer: Global Action for Skilled Attendants
Jor Pregnant Women. WHO/RHR/02.17. Geneva: WHO.
Wdhams, A. I9K7. "Health Economics: The Cheerful Face of the Dismal
Science. In Health and Economics, ed. A. Williams. 1-11. Basingstoke
and London: Macmillan.
. 2004a. “Global Monitoring and Evaluation, Proportion of Births
Subre^n
Sk,lled Heahh Personnel: Global, Regional, and
Sub egmnal Estimates Geneva, WHO, Department of Reproductive
Health and Research, http://www.who.int/reproductive-health/
global_momtoring/data_regions.html.
UNICEF, UNFPA. Geneva: WHO.
Developed by WHO,
7
Winikofh B and M. Sullivan. 1987. "Assessing the Role of Family PlannlR8m.
MatCrnal Mortali,y” Studics
inning
Yasmin, S.» D Osrin, E. Paul, and A. Costello. 2001."Neonatal Mortality of
Low Birth-Weight Infants in Bangladesh.” Bulletin of the World Health
Organization 79 (7): 608-14.
4
Maternal and Perinatal Conditions I 31
Position: 1368 (5 views)